Bioluminescent imaging in induced mouse models of endometriosis reveals differences in four model variations
- PMID: 34382636
- PMCID: PMC8419713
- DOI: 10.1242/dmm.049070
Bioluminescent imaging in induced mouse models of endometriosis reveals differences in four model variations
Abstract
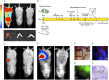
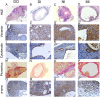
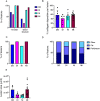
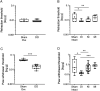
Our understanding of the aetiology and pathophysiology of endometriosis remains limited. Disease modelling in the field is problematic as many versions of induced mouse models of endometriosis exist. We integrated bioluminescent imaging of 'lesions' generated using luciferase-expressing donor mice. We compared longitudinal bioluminescence and histology of lesions, sensory behaviour of mice with induced endometriosis and the impact of the gonadotropin-releasing hormone antagonist Cetrorelix on lesion regression and sensory behaviour. Four models of endometriosis were tested. We found that the nature of the donor uterine material was a key determinant of how chronic the lesions were, as well as their cellular composition. The severity of pain-like behaviour also varied across models. Although Cetrorelix significantly reduced lesion bioluminescence in all models, it had varying impacts on pain-like behaviour. Collectively, our results demonstrate key differences in the progression of the 'disease' across different mouse models of endometriosis. We propose that validation and testing in multiple models, each of which may be representative of the different subtypes/heterogeneity observed in women, should become a standard approach to discovery science in the field of endometriosis.
Keywords: Endometriosis; GnRH antagonist; Lesion; Pain.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Cousins, F. L., Murray, A., Esnal, A., Gibson, D. A., Critchley, H. O. D. and Saunders, P. T. K. (2014). Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS ONE 9, e86378. 10.1371/journal.pone.0086378 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

