Light-Switchable Buffers
- PMID: 34382726
- PMCID: PMC8518091
- DOI: 10.1002/anie.202109250
Light-Switchable Buffers
Abstract
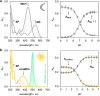
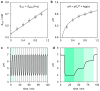
A visible light-switchable buffer system based on a merocyanine photoacid is presented. Para-substitution of the indolium side with a methoxy group affords a compound suitable for making hydrolytically stable aqueous buffers whose pH can be tuned between 7 and 4 using 500 nm light.
Keywords: buffers; molecular switches; photoacidity; protonated merocyanines; water.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sorensen S. P. L., Biochem. Z. 1909, 21, 131–304.
-
- Stoll V. S., Blanchard J. S., Methods in Enzymology, Vol. 182 (Ed.: Deutscher M. P.), Academic Press, New York, 1990, pp. 24–38. - PubMed
-
- Urbansky E. T., Schock M. R., J. Chem. Educ. 2000, 77, 1640–1644.
-
- None
-
- Klajn R., Chem. Soc. Rev. 2014, 43, 148–184; - PubMed
Publication types
LinkOut - more resources
Full Text Sources

