Cetuximab Rechallenge Plus Avelumab in Pretreated Patients With RAS Wild-type Metastatic Colorectal Cancer: The Phase 2 Single-Arm Clinical CAVE Trial
- PMID: 34382998
- PMCID: PMC8531995
- DOI: 10.1001/jamaoncol.2021.2915
Cetuximab Rechallenge Plus Avelumab in Pretreated Patients With RAS Wild-type Metastatic Colorectal Cancer: The Phase 2 Single-Arm Clinical CAVE Trial
Abstract
Importance: Rechallenge therapy with anti-epidermal growth factor receptor (EGFR) drugs has been suggested in patients with chemo-refractory RAS wild-type (WT) metastatic colorectal cancer (mCRC) after initial response to anti-EGFR-based first-line treatment. The association of treatment with cetuximab plus avelumab with overall survival (OS) may be worthy of investigation in this setting.
Objective: To assess the efficacy and safety of cetuximab rechallenge therapy plus avelumab.
Design, setting, and participants: This single-arm, multicenter phase 2 trial enrolled patients from August 2018 to February 2020. Eligible patients with RAS WT mCRC had a complete or partial response to first-line chemotherapy plus anti-EGFR drugs, developed acquired resistance, and failed second-line therapy. Baseline circulating tumor DNA (ctDNA) for KRAS, NRAS, BRAF, and EGFR-S492R mutation analysis was done.
Interventions: Patients received avelumab (10 mg/kg every 2 weeks) and cetuximab (400 mg/m2 and, subsequently, 250 mg/m2 weekly) until disease progression or unacceptable toxic effects.
Main outcomes and measures: The primary end point was OS. Secondary end points were progression-free survival (PFS), overall response rate (ORR), and safety.
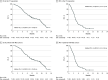
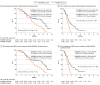
Results: Seventy-seven patients were enrolled (42 men, 35 women; median age, 63 years); 71 had microsatellite stable tumors (MSS), 3 microsatellite instability-high tumors (MSI-H), 3 unknown. The study met the primary end point, with median OS (mOS) of 11.6 months (95% CI, 8.4-14.8 months). Median PFS (mPFS) was 3.6 months (95% CI, 3.2-4.1 months). Common grade-3 adverse events were cutaneous eruption, 11 (14%), and diarrhea, 3 (4%). For 67 of 77 (87%) patients, baseline analysis of plasma circulating tumor DNA (ctDNA) for KRAS, NRAS, BRAF, and EGFR-S492R variations was feasible. Forty-eight patients had WT disease, whereas 19 had mutations. Patients with RAS/BRAF WT ctDNA had mOS of 17.3 months (95% CI, 12.5-22.0 months) compared with 10.4 months (95% CI, 7.2-13.6 months) in patients with mutated ctDNA (hazard ratio [HR], 0.49; 95% CI, 0.27-0.90; P = .02). The mPFS was 4.1 months (95% CI, 2.9-5.2 months) in RAS/BRAF WT patients compared with 3.0 months (95% CI, 2.6-3.5 months) in patients with mutated ctDNA (HR, 0.42; 95% CI, 0.23-0.75; P = .004).
Conclusions and relevance: The findings of this single-arm phase 2 trial suggest that cetuximab plus avelumab is an active, well tolerated rechallenge therapy in RAS WT mCRC. Plasma ctDNA analysis before treatment may allow selection of patients who could benefit.
Trial registration: ClinicalTrials.gov Identifier: NCT04561336.
Conflict of interest statement
Figures
References
-
- Heinemann V, von Weikersthal LF, Decker T, et al. . FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065-1075. doi:10.1016/S1470-2045(14)70330-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

