Evaluating longitudinal therapy effects via the North Star Ambulatory Assessment
- PMID: 34383312
- PMCID: PMC9290940
- DOI: 10.1002/mus.27396
Evaluating longitudinal therapy effects via the North Star Ambulatory Assessment
Abstract
Introduction/aims: In comparative studies, treatment effects are typically evaluated at a specific time point. When data are collected periodically, an alternative, clinically meaningful approach could be used to assess the totality of treatment effects. We applied a well-developed analytical procedure for evaluating longitudinal treatment effects using North Star Ambulatory Assessment (NSAA) data for illustration.
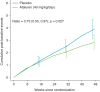
Methods: The NSAA comprises 17 scorable items/outcomes that measure changes in motor function. Using NSAA data from the published ataluren phase 3, randomized, placebo-controlled trial (NCT01826487), cumulative counts of failures to perform each item (transition from 2/1 [able/impaired] to 0 [unable]) were collected at specified time points for each patient over 48 wk. Treatment group-wise mean cumulative item failure count curves were constructed, comparing ataluren versus placebo and deflazacort versus prednisone/prednisolone among placebo-treated patients. The steeper the curve, the worse the outcome. A clinically meaningful summary of the between-group difference was provided for each comparison.
Results: The curve was uniformly steeper for placebo than ataluren after 16 wk and for prednisone/prednisolone than deflazacort after 8 wk. The two curves in each comparison continued to diverge thereafter, indicating sustained treatment benefits over time. Using a unique analytical approach, cumulative failure rates were reduced, on average, by 27% for ataluren versus placebo (rate ratio, 0.73; 95% confidence interval [CI], 0.55-0.97; p = .027) and 28% for deflazacort versus prednisone/prednisolone (rate ratio, 0.72; 95% CI, 0.53-0.96; p = .028).
Discussion: Unlike fixed-time analyses, this analytical approach enabled demonstration of cumulative, longitudinal treatment effects over time using repeatedly measured NSAA observations.
Keywords: Duchenne muscular dystrophy; efficacy; motor function; outcome measure; treatment.
© 2021 The Authors. Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
Craig M. McDonald has acted as a consultant on clinical trials of DMD for Astellas, Capricor, Catabasis, Edgewise Therapeutics, Epirium Bio (formerly Cardero Therapeutics), FibroGen, Italfarmaco, Pfizer, PTC Therapeutics, Santhera Pharmaceuticals and Sarepta Therapeutics. He has received research support for clinical trials from Capricor, Catabasis, Italfarmaco, Pfizer, PTC Therapeutics, Santhera Pharmaceuticals and Sarepta Therapeutics. Lee‐Jen Wei has acted as a consultant for Johnson and Johnson, Merck and co., Pfizer, PTC Therapeutics and Sarepta Therapeutics. Kevin M. Flanigan has acted as a consultant for Apic Bio, Audentes Therapeutics, Dynacure, Italfarmaco, Marathon Pharmaceuticals, PTC Therapeutics, Santhera Pharmaceuticals, Sarepta Therapeutics, Tivorsan Pharmaceuticals, and 4D Molecular Therapeutics; has been a clinical trial investigator for Abeona Therapeutics, Akashi Therapeutics BioMarin and Sarepta Therapeutics; has received grants from Beauhawks Foundation and CureDuchenne; and has received royalty payment from Audentes Therapeutics. Gary Elfring and Panayiota Trifillis are employees of PTC Therapeutics. Francesco Muntoni has received consulting fees from AveXis, Biogen, Dyne Therapeutics, Capricor, Catabasis, Novartis, Pfizer, PTC Therapeutics, Roche, Santhera Pharmaceuticals, Sarepta Therapeutics and Wave Therapeutics, and is supported by the National Institute of Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. Medical writing and editorial support were funded by PTC Therapeutics Ltd.
Figures

References
-
- Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77‐93. - PubMed
-
- Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919‐928. - PubMed

