Multi- and Transgenerational Outcomes of an Exposure to a Mixture of Endocrine-Disrupting Chemicals (EDCs) on Puberty and Maternal Behavior in the Female Rat
- PMID: 34383603
- PMCID: PMC8360047
- DOI: 10.1289/EHP8795
Multi- and Transgenerational Outcomes of an Exposure to a Mixture of Endocrine-Disrupting Chemicals (EDCs) on Puberty and Maternal Behavior in the Female Rat
Abstract
Background: The effects of endocrine-disrupting chemicals (EDCs) on fertility and reproductive development represent a rising concern in modern societies. Although the neuroendocrine control of sexual maturation is a major target of EDCs, little is known about the potential role of the hypothalamus in puberty and ovulation disruption transmitted across generations.
Objectives: We hypothesized that developmental exposure to an environmentally relevant dose of EDC mixture could induce multi- and/or transgenerational alterations of sexual maturation and maternal care in female rats through epigenetic reprograming of the hypothalamus. We investigated the transmission of a disrupted reproductive phenotype via the maternal germline or via nongenomic mechanisms involving maternal care.
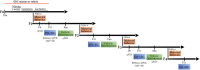
Methods: Adult female Wistar rats were exposed prior to and during gestation and until the end of lactation to a mixture of the following 13 EDCs: di-n-butyl phthalate (DnBP), di(2-ethylhexyl) phthalate (DEHP), bisphenol A (BPA), vinclozolin, prochloraz, procymidone, linuron, epoxynaxole, dichlorodiphenyldichloroethylene, octyl methoxynimmate, 4-methylbenzylidene camphor (4-MBC), butylparaben, and acetaminophen. Perinatally exposed offspring (F1) were mated with unexposed males to generate germ cell (F2) and transgenerationally exposed (F3 and F4) females. Sexual maturation, maternal behavior, and hypothalamic targets of exposure were studied across generations.
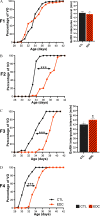
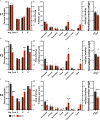
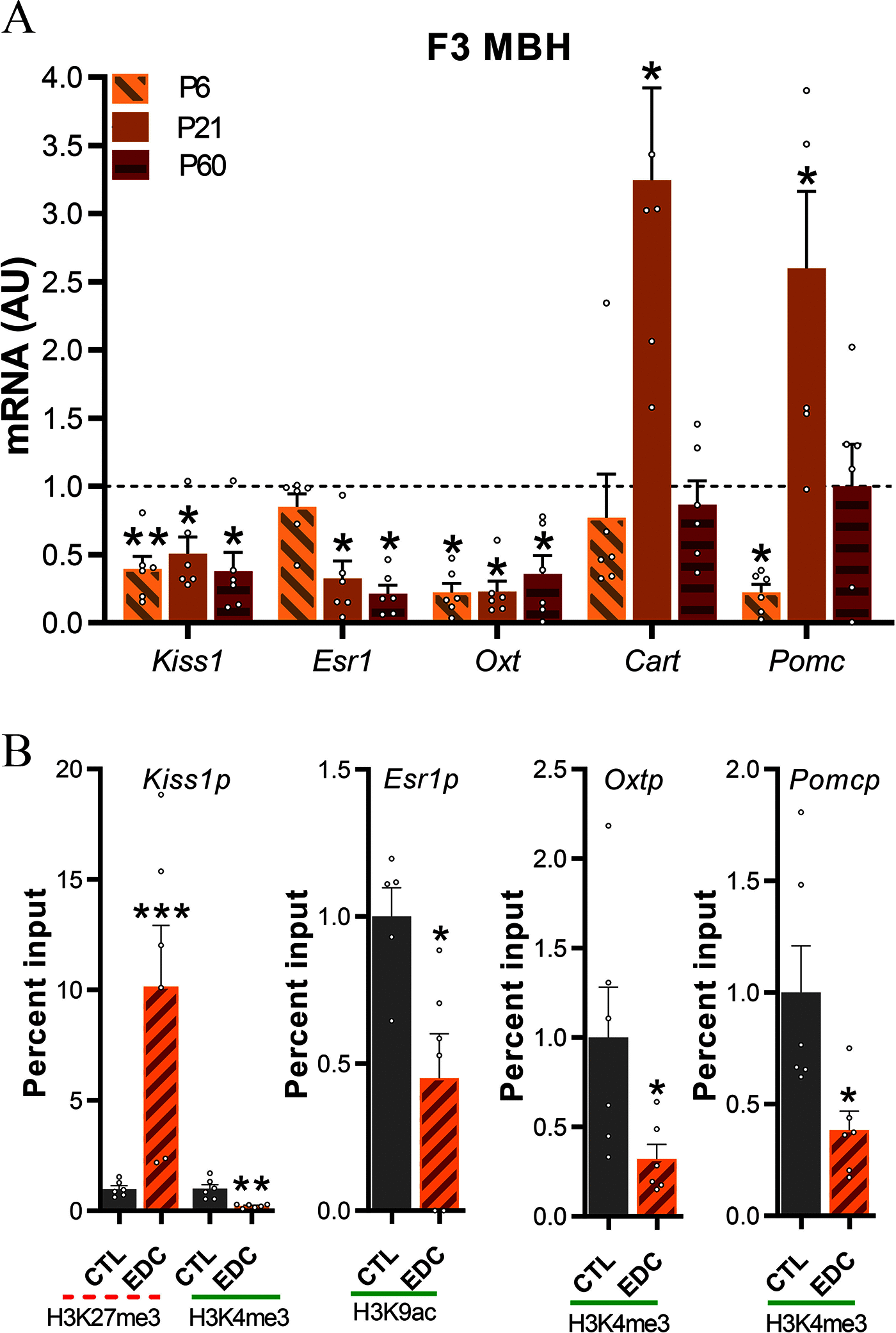
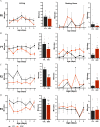
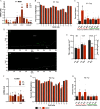
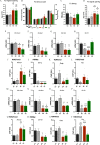
Results: Germ cell (F2) and transgenerationally (F3) EDC-exposed females, but not F1, displayed delayed pubertal onset and altered folliculogenesis. We reported a transgenerational alteration of key hypothalamic genes controlling puberty and ovulation (Kiss1, Esr1, and Oxt), and we identified the hypothalamic polycomb group of epigenetic repressors as actors of this mechanism. Furthermore, we found a multigenerational reduction of maternal behavior (F1-F3) induced by a loss in hypothalamic dopaminergic signaling. Using a cross-fostering paradigm, we identified that the reduction in maternal phenotype was normalized in EDC-exposed pups raised by unexposed dams, but no reversal of the pubertal phenotype was achieved.
Discussion: Rats developmentally exposed to an EDC mixture exhibited multi- and transgenerational disruption of sexual maturation and maternal care via hypothalamic epigenetic reprogramming. These results raise concerns about the impact of EDC mixtures on future generations. https://doi.org/10.1289/EHP8795.
Figures
Comment in
-
Invited Perspective: Does Developmental Adaptation Pose Risks with Changing Toxicant Exposures?Environ Health Perspect. 2021 Aug;129(8):81302. doi: 10.1289/EHP9560. Epub 2021 Aug 12. Environ Health Perspect. 2021. PMID: 34383604 Free PMC article. No abstract available.
References
-
- Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. 2009. Neonatal bisphenol-A exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod 81(4):690–699, PMID: 19535786, 10.1095/biolreprod.109.078261. - DOI - PMC - PubMed
-
- Arambula SE, Belcher SM, Planchart A, Turner SD, Patisaul HB. 2016. Impact of low dose oral exposure to bisphenol A (BPA) on the neonatal rat hypothalamic and hippocampal transcriptome: a CLARITY-BPA Consortium study. Endocrinology 157(10):3856–3872, PMID: 27571134, 10.1210/en.2016-1339. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
