A Modified Public Health Automated Case Event Reporting Platform for Enhancing Electronic Laboratory Reports With Clinical Data: Design and Implementation Study
- PMID: 34383669
- PMCID: PMC8387889
- DOI: 10.2196/26388
A Modified Public Health Automated Case Event Reporting Platform for Enhancing Electronic Laboratory Reports With Clinical Data: Design and Implementation Study
Abstract
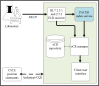
Background: Public health reporting is the cornerstone of public health practices that inform prevention and control strategies. There is a need to leverage advances made in the past to implement an architecture that facilitates the timely and complete public health reporting of relevant case-related information that has previously not easily been available to the public health community. Electronic laboratory reporting (ELR) is a reliable method for reporting cases to public health authorities but contains very limited data. In an earlier pilot study, we designed the Public Health Automated Case Event Reporting (PACER) platform, which leverages existing ELR infrastructure as the trigger for creating an electronic case report. PACER is a FHIR (Fast Health Interoperability Resources)-based system that queries the electronic health record from where the laboratory test was requested to extract expanded additional information about a case.
Objective: This study aims to analyze the pilot implementation of a modified PACER system for electronic case reporting and describe how this FHIR-based, open-source, and interoperable system allows health systems to conduct public health reporting while maintaining the appropriate governance of the clinical data.
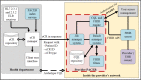
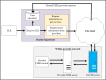
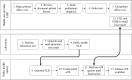
Methods: ELR to a simulated public health department was used as the trigger for a FHIR-based query. Predetermined queries were translated into Clinical Quality Language logics. Within the PACER environment, these Clinical Quality Language logical statements were managed and evaluated against the providers' FHIR servers. These predetermined logics were filtered, and only data relevant to that episode of the condition were extracted and sent to simulated public health agencies as an electronic case report. Design and testing were conducted at the Georgia Tech Research Institute, and the pilot was deployed at the Medical University of South Carolina. We evaluated this architecture by examining the completeness of additional information in the electronic case report, such as patient demographics, medications, symptoms, and diagnoses. This additional information is crucial for understanding disease epidemiology, but existing electronic case reporting and ELR architectures do not report them. Therefore, we used the completeness of these data fields as the metrics for enriching electronic case reports.
Results: During the 8-week study period, we identified 117 positive test results for chlamydia. PACER successfully created an electronic case report for all 117 patients. PACER extracted demographics, medications, symptoms, and diagnoses from 99.1% (116/117), 72.6% (85/117), 70.9% (83/117), and 65% (76/117) of the cases, respectively.
Conclusions: PACER deployed in conjunction with electronic laboratory reports can enhance public health case reporting with additional relevant data. The architecture is modular in design, thereby allowing it to be used for any reportable condition, including evolving outbreaks. PACER allows for the creation of an enhanced and more complete case report that contains relevant case information that helps us to better understand the epidemiology of a disease.
Keywords: EHR; chlamydia; electronic case reporting; electronic health records; electronic laboratory reporting; fast healthcare interoperability resources; gonorrhea; health information interoperability; public health surveillance; sexually transmitted diseases.
©Ninad Mishra, Jon Duke, Saugat Karki, Myung Choi, Michael Riley, Andrey V Ilatovskiy, Marla Gorges, Leslie Lenert. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 11.08.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
Automating Case Reporting of Chlamydia and Gonorrhea to Public Health Authorities in Illinois Clinics: Implementation and Evaluation of Findings.JMIR Public Health Surveill. 2023 Mar 14;9:e38868. doi: 10.2196/38868. JMIR Public Health Surveill. 2023. PMID: 36917153 Free PMC article.
-
Integration of FHIR to Facilitate Electronic Case Reporting: Results from a Pilot Study.Stud Health Technol Inform. 2019 Aug 21;264:940-944. doi: 10.3233/SHTI190362. Stud Health Technol Inform. 2019. PMID: 31438062
-
Data quality and timeliness analysis for post-vaccination adverse event cases reported through healthcare data exchange to FDA BEST pilot platform.Front Public Health. 2024 Jul 8;12:1379973. doi: 10.3389/fpubh.2024.1379973. eCollection 2024. Front Public Health. 2024. PMID: 39040857 Free PMC article.
-
Electronic Health Record Use in Public Health Infectious Disease Surveillance, USA, 2018-2019.Curr Infect Dis Rep. 2019 Aug 26;21(10):32. doi: 10.1007/s11908-019-0694-5. Curr Infect Dis Rep. 2019. PMID: 31451945 Review.
-
Fast Healthcare Interoperability Resources (FHIR) for Interoperability in Health Research: Systematic Review.JMIR Med Inform. 2022 Jul 19;10(7):e35724. doi: 10.2196/35724. JMIR Med Inform. 2022. PMID: 35852842 Free PMC article. Review.
Cited by
-
An economic evaluation of the expansion of electronic case reporting in an academic healthcare setting.JAMIA Open. 2024 Jan 12;7(1):ooad102. doi: 10.1093/jamiaopen/ooad102. eCollection 2024 Apr. JAMIA Open. 2024. PMID: 38223408 Free PMC article.
-
Extracting laboratory test information from paper-based reports.BMC Med Inform Decis Mak. 2023 Nov 6;23(1):251. doi: 10.1186/s12911-023-02346-6. BMC Med Inform Decis Mak. 2023. PMID: 37932733 Free PMC article.
-
Population-Based Linked Longitudinal Surveillance of Pregnant People and Their Infants: A Critical Resource for Emerging, Re-Emerging, and Persistent Threats.J Womens Health (Larchmt). 2023 Jan;32(1):1-9. doi: 10.1089/jwh.2022.0419. Epub 2022 Nov 28. J Womens Health (Larchmt). 2023. PMID: 36454196 Free PMC article.
-
Evaluation of Automated Processing of Electronically Reported Serological Tests for Syphilis Using Current and Historical Syphilis Results Compared With Traditional Reactor Grid Processing in Florida.Sex Transm Dis. 2024 Jun 1;51(6):420-424. doi: 10.1097/OLQ.0000000000001952. Epub 2024 Feb 19. Sex Transm Dis. 2024. PMID: 38372524 Free PMC article.
-
Development of a Standardized, Laboratory Result-Based Hepatitis C Virus Clearance Cascade for Public Health Jurisdictions.Public Health Rep. 2024 Mar-Apr;139(2):149-153. doi: 10.1177/00333549231170044. Epub 2023 May 4. Public Health Rep. 2024. PMID: 37140162 Free PMC article. No abstract available.
References
-
- Wharton M, Chorba TL, Vogt RL, Morse DL, Buehler JW. Case definitions for public health surveillance. MMWR Recomm Rep. 1990 Oct 19;39(RR-13):1–43. http://www.cdc.gov/mmwr/preview/mmwrhtml/00025629.htm - PubMed
-
- Centers for Disease Control and Prevention Case definitions for infectious conditions under public health surveillance. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1997 May 02;46(RR-10):1–55. http://www.cdc.gov/mmwr/preview/mmwrhtml/00047449.htm - PubMed
-
- Mac Kenzie WR, Davidson AJ, Wiesenthal A, Engel JP, Turner K, Conn L, Becker SJ, Moffatt S, Groseclose SL, Jellison J, Stinn J, Garrett NY, Helmus L, Harmon B, Richards CL, Lumpkin JR, Iademarco MF. The Promise of Electronic Case Reporting. Public Health Rep. 2016 Nov;131(6):742–746. doi: 10.1177/0033354916670871. http://europepmc.org/abstract/MED/28123218 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

