Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥65 Years - COVID-NET, 13 States, February-April 2021
- PMID: 34383730
- PMCID: PMC8360274
- DOI: 10.15585/mmwr.mm7032e3
Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥65 Years - COVID-NET, 13 States, February-April 2021
Abstract
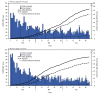
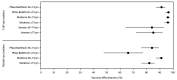
Clinical trials of COVID-19 vaccines currently authorized for emergency use in the United States (Pfizer-BioNTech, Moderna, and Janssen [Johnson & Johnson]) indicate that these vaccines have high efficacy against symptomatic disease, including moderate to severe illness (1-3). In addition to clinical trials, real-world assessments of COVID-19 vaccine effectiveness are critical in guiding vaccine policy and building vaccine confidence, particularly among populations at higher risk for more severe illness from COVID-19, including older adults. To determine the real-world effectiveness of the three currently authorized COVID-19 vaccines among persons aged ≥65 years during February 1-April 30, 2021, data on 7,280 patients from the COVID-19-Associated Hospitalization Surveillance Network (COVID-NET) were analyzed with vaccination coverage data from state immunization information systems (IISs) for the COVID-NET catchment area (approximately 4.8 million persons). Among adults aged 65-74 years, effectiveness of full vaccination in preventing COVID-19-associated hospitalization was 96% (95% confidence interval [CI] = 94%-98%) for Pfizer-BioNTech, 96% (95% CI = 95%-98%) for Moderna, and 84% (95% CI = 64%-93%) for Janssen vaccine products. Effectiveness of full vaccination in preventing COVID-19-associated hospitalization among adults aged ≥75 years was 91% (95% CI = 87%-94%) for Pfizer-BioNTech, 96% (95% CI = 93%-98%) for Moderna, and 85% (95% CI = 72%-92%) for Janssen vaccine products. COVID-19 vaccines currently authorized in the United States are highly effective in preventing COVID-19-associated hospitalizations in older adults. In light of real-world data demonstrating high effectiveness of COVID-19 vaccines among older adults, efforts to increase vaccination coverage in this age group are critical to reducing the risk for COVID-19-related hospitalization.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Evan J. Anderson reports grants from Pfizer, Merck, PaxVax, Micron, Sanofi-Pasteur, Janssen, MedImmune, and GSK; personal fees from Sanofi-Pasteur, Pfizer, Medscape, Kentucky Bioprocessing, Inc, Janssen, outside the submitted work; and his institution has also received funding from NIH to conduct clinical trials of Moderna and Janssen COVID-19 vaccines. Sue Kim reports grants from Michigan Department of Health and Human Services, during the conduct of the study. William Schaffner reports personal fees from VBI Vaccines, outside the submitted work. Jessica Shiltz reports grants from Council for State and Territorial Epidemiologists during the conduct of the study. No other potential conflicts of interest were disclosed.
Figures
References
-
- Tenforde MW, Olson SM, Self WH, et al.; IVY Network; HAIVEN Investigators. Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep 2021;70:674–9. 10.15585/mmwr.mm7018e1 - DOI - PMC - PubMed

