Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis
- PMID: 34383750
- PMCID: PMC8389849
- DOI: 10.1371/journal.pmed.1003735
Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis
Erratum in
-
Correction: Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis.PLoS Med. 2021 Oct 13;18(10):e1003825. doi: 10.1371/journal.pmed.1003825. eCollection 2021 Oct. PLoS Med. 2021. PMID: 34644309 Free PMC article.
Abstract
Background: SARS-CoV-2 antigen rapid diagnostic tests (Ag-RDTs) are increasingly being integrated in testing strategies around the world. Studies of the Ag-RDTs have shown variable performance. In this systematic review and meta-analysis, we assessed the clinical accuracy (sensitivity and specificity) of commercially available Ag-RDTs.
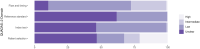
Methods and findings: We registered the review on PROSPERO (registration number: CRD42020225140). We systematically searched multiple databases (PubMed, Web of Science Core Collection, medRvix, bioRvix, and FIND) for publications evaluating the accuracy of Ag-RDTs for SARS-CoV-2 up until 30 April 2021. Descriptive analyses of all studies were performed, and when more than 4 studies were available, a random-effects meta-analysis was used to estimate pooled sensitivity and specificity in comparison to reverse transcription polymerase chain reaction (RT-PCR) testing. We assessed heterogeneity by subgroup analyses, and rated study quality and risk of bias using the QUADAS-2 assessment tool. From a total of 14,254 articles, we included 133 analytical and clinical studies resulting in 214 clinical accuracy datasets with 112,323 samples. Across all meta-analyzed samples, the pooled Ag-RDT sensitivity and specificity were 71.2% (95% CI 68.2% to 74.0%) and 98.9% (95% CI 98.6% to 99.1%), respectively. Sensitivity increased to 76.3% (95% CI 73.1% to 79.2%) if analysis was restricted to studies that followed the Ag-RDT manufacturers' instructions. LumiraDx showed the highest sensitivity, with 88.2% (95% CI 59.0% to 97.5%). Of instrument-free Ag-RDTs, Standard Q nasal performed best, with 80.2% sensitivity (95% CI 70.3% to 87.4%). Across all Ag-RDTs, sensitivity was markedly better on samples with lower RT-PCR cycle threshold (Ct) values, i.e., <20 (96.5%, 95% CI 92.6% to 98.4%) and <25 (95.8%, 95% CI 92.3% to 97.8%), in comparison to those with Ct ≥ 25 (50.7%, 95% CI 35.6% to 65.8%) and ≥30 (20.9%, 95% CI 12.5% to 32.8%). Testing in the first week from symptom onset resulted in substantially higher sensitivity (83.8%, 95% CI 76.3% to 89.2%) compared to testing after 1 week (61.5%, 95% CI 52.2% to 70.0%). The best Ag-RDT sensitivity was found with anterior nasal sampling (75.5%, 95% CI 70.4% to 79.9%), in comparison to other sample types (e.g., nasopharyngeal, 71.6%, 95% CI 68.1% to 74.9%), although CIs were overlapping. Concerns of bias were raised across all datasets, and financial support from the manufacturer was reported in 24.1% of datasets. Our analysis was limited by the included studies' heterogeneity in design and reporting.
Conclusions: In this study we found that Ag-RDTs detect the vast majority of SARS-CoV-2-infected persons within the first week of symptom onset and those with high viral load. Thus, they can have high utility for diagnostic purposes in the early phase of disease, making them a valuable tool to fight the spread of SARS-CoV-2. Standardization in conduct and reporting of clinical accuracy studies would improve comparability and use of data.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: CMD is a member of the Editorial Board of PLOS Medicine.
Figures



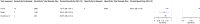

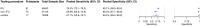
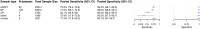

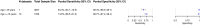
References
-
- World Health Organization. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays: interim guidance, 11 September 2020. WHO/2019-nCoV/Antigen_Detection/2020.1. Geneva: World Health Organization; 2020.
-
- Federal Institute for Drugs and Medical Devices. Antigen-Tests auf SARS-CoV-2. Bonn: Federal Institute for Drugs and Medical Devices; 2021 [cited 2021 Jun 7]. https://www.bfarm.de/DE/Medizinprodukte/Antigentests/_node.html.
-
- Krüger LJ, Gaeddert M, Köppel L, Brümmer LE, Gottschalk C, Miranda IB, et al. Evaluation of the accuracy, ease of use and limit of detection of novel, rapid, antigen-detecting point-of-care diagnostics for SARS-CoV-2. medRxiv. 2020Oct4. doi: 10.1101/2020.10.01.20203836 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

