Chemical control and insecticide resistance status of sand fly vectors worldwide
- PMID: 34383751
- PMCID: PMC8360369
- DOI: 10.1371/journal.pntd.0009586
Chemical control and insecticide resistance status of sand fly vectors worldwide
Abstract
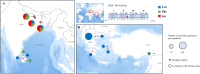
Background: Phlebotomine sand flies are prominent vectors of Leishmania parasites that cause leishmaniasis, which comes second to malaria in terms of parasitic causative fatalities globally. In the absence of human vaccines, sand fly chemical-based vector control is a key component of leishmaniasis control efforts.
Methods and findings: We performed a literature review on the current interventions, primarily, insecticide-based used for sand fly control, as well as the global insecticide resistance (IR) status of the main sand fly vector species. Indoor insecticidal interventions, such as residual spraying and treated bed nets are the most widely deployed, while several alternative control strategies are also used in certain settings and/or are under evaluation. IR has been sporadically detected in sand flies in India and other regions, using non-standardized diagnostic bioassays. Molecular studies are limited to monitoring of known pyrethroid resistance mutations (kdr), which are present at high frequencies in certain regions.
Conclusions: As the leishmaniasis burden remains a major problem at a global scale, evidence-based rational use of insecticidal interventions is required to meet public health demands. Standardized bioassays and molecular markers are a prerequisite for this task, albeit are lagging behind. Experiences from other disease vectors underscore the need for the implementation of appropriate IR management (IRM) programs, in the framework of integrated vector management (IVM). The implementation of alternative strategies seems context- and case-specific, with key eco-epidemiological parameters yet to be investigated. New biotechnology-based control approaches might also come into play in the near future to further reinforce sand fly/leishmaniasis control efforts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organization. Leishmaniasis. 2020. Available from: https://www.who.int/health-topics/leishmaniasis#tab=tab_1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

