Evaluation of a point-of-care diagnostic to identify glucose-6-phosphate dehydrogenase deficiency in Brazil
- PMID: 34383774
- PMCID: PMC8384181
- DOI: 10.1371/journal.pntd.0009649
Evaluation of a point-of-care diagnostic to identify glucose-6-phosphate dehydrogenase deficiency in Brazil
Abstract
Background: Glucose-6-phosphate dehydrogenase (G6PD) deficiency is a common enzyme deficiency, prevalent in many malaria-endemic countries. G6PD-deficient individuals are susceptible to hemolysis during oxidative stress, which can occur from exposure to certain medications, including 8-aminoquinolines used to treat Plasmodium vivax malaria. Accordingly, access to point-of-care (POC) G6PD testing in Brazil is critical for safe treatment of P. vivax malaria.
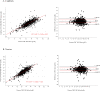
Methodology/principal findings: This study evaluated the performance of the semi-quantitative, POC STANDARD G6PD Test (SD Biosensor, Republic of Korea). Participants were recruited at clinics and through an enriched sample in Manaus and Porto Velho, Brazil. G6PD and hemoglobin measurements were obtained from capillary samples at the POC using the STANDARD and HemoCue 201+ (HemoCue AB, Sweden) tests. A thick blood slide was prepared for malaria microscopy. At the laboratories, the STANDARD and HemoCue tests were repeated on venous samples and a quantitative spectrophotometric G6PD reference assay was performed (Pointe Scientific, Canton, MI). G6PD was also assessed by fluorescent spot test. In Manaus, a complete blood count was performed. Samples were analyzed from 1,736 participants. In comparison to spectrophotometry, the STANDARD G6PD Test performed equivalently in determining G6PD status in venous and capillary specimens under varied operating temperatures. Using the manufacturer-recommended reference value thresholds, the test's sensitivity at the <30% threshold on both specimen types was 100% (95% confidence interval [CI] venous 93.6%-100.0%; capillary 93.8%-100.0%). Specificity was 98.6% on venous specimens (95% CI 97.9%-99.1%) and 97.8% on capillary (95% CI 97.0%-98.5%). At the 70% threshold, the test's sensitivity was 96.9% on venous specimens (95% CI 83.8%-99.9%) and 94.3% on capillary (95% CI 80.8%-99.3%). Specificity was 96.5% (95% CI 95.0%-97.6%) and 92.3% (95% CI 90.3%-94.0%) on venous and capillary specimens, respectively.
Conclusion/significance: The STANDARD G6PD Test is a promising tool to aid in POC detection of G6PD deficiency in Brazil.
Trial registration: This study was registered with ClinicalTrials.gov (identifier: NCT04033640).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

