Outcomes in 1096 patients with severe thrombotic thrombocytopenic purpura before the Caplacizumab era
- PMID: 34383822
- PMCID: PMC8360509
- DOI: 10.1371/journal.pone.0256024
Outcomes in 1096 patients with severe thrombotic thrombocytopenic purpura before the Caplacizumab era
Abstract
Introduction: Thrombotic thrombocytopenic purpura (TTP) is a diagnostic and therapeutic emergency. Therapeutic plasma exchange (TPE) combined with immunosuppression has been the cornerstone of the initial management. To produce optimal benefits, emerging treatments must be used against a background of best standard of care. Clarifying current uncertainties is therefore crucial.
Methods: The objective of this study was to analyze a large high-quality database (Marketscan) of TTP patients managed between 2005 and 2014, in the pre-caplacizumab era, in order to assess the impact of time to first TPE and use of first-line rituximab on mortality, and whether mortality declines over time.
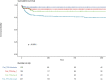
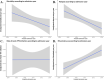
Results: Among the 1096 included patients (median age 46 [IQR 35-55], 70% female), 28.8% received TPE before day 2 in the ICU. Hospital mortality was 7.6% (83 deaths). Mortality was independently associated with older age (hazard ratio [HR], 1.024/year; 95% confidence interval [95%CI], [1.009-1.040]), diagnosis of sepsis (HR, 2.360; 95%CI [1.552-3.588]), and the need for mechanical ventilation (HR, 4.103; 95%CI, [2.749-6.126]). Factors independently associated with lower mortality were TPE at ICU admission (HR, 0.284; 95%CI, [0.112-0.717]), TPE within one day after ICU admission (HR, 0.449; 95%CI, [0.275-0.907]), and early rituximab therapy (HR, 0.229; 95% CI, [0.111-0.471]). Delayed TPE was associated with significantly higher costs.
Conclusions: Immediate TPE and early rituximab are associated with improved survival in TTP patients. Improved treatments have led to a decline in mortality over time, and alternate outcome variables such as the use of hospital resources or longer term outcomes therefore need to be considered.
Conflict of interest statement
“I have read the journal`s policy and the authors of this manuscript have the following competing interests: EA has received fees for lectures from Gilead, Pfizer, Baxter, and Alexion. His research group has been supported by Ablynx, Fisher & Payckle, Jazz Pharma, and MSD. MD has received fees for lectures from Gilead, Astelas, and MSD. His institution has received a research grant from MSD. All other authors have no conflict of interest to disclose. This does not alter our adherence to PLOS ONE policies on sharing data and materials
Figures
References
-
- Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, et al.. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100(12):4033–9. Epub 2002/10/24. doi: 10.1182/blood-2002-05-1401 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

