Functional Vision in the Real-World Environment With a Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis
- PMID: 34383875
- PMCID: PMC8362639
- DOI: 10.1167/tvst.10.10.7
Functional Vision in the Real-World Environment With a Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis
Abstract
Purpose: In a clinical trial (NCT03406416) of a second-generation (44-channel) suprachoroidal retinal prosthesis implanted in subjects with late-stage retinitis pigmentosa (RP), we assessed performance in real-world functional visual tasks and emotional well-being.
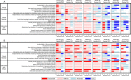
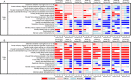
Methods: The Functional Low-Vision Observer Rated Assessment (FLORA) and Impact of Vision Impairment-Very Low Vision (IVI-VLV) instruments were administered to four subjects before implantation and after device fitting. The FLORA contains 13 self-reported and 35 observer-reported items ranked for ease of conducting task (impossible-easy, central tendency given as mode). The IVI-VLV instrument quantified the impact of low vision on daily activities and emotional well-being.
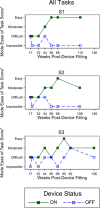
Results: Three subjects completed the FLORA for two years after device fitting; the fourth subject ceased participation in the FLORA after fitting for reasons unrelated to the device. For all subjects at each post-fitting visit, the mode ease of task with device ON was better or equal to device OFF. Ease of task improved over the first six months with device ON, then remained stable. Subjects reported improvements in mobility, functional vision, and quality of life with device ON. The IVI-VLV suggested self-assessed vision-related quality of life was not impacted by device implantation or usage.
Conclusions: Subjects demonstrated sustained improved ease of task scores with device ON compared to OFF, indicating the device has a positive impact in the real-world setting.
Translational relevance: Our suprachoroidal retinal prosthesis shows potential utility in everyday life, by enabling an increased environmental awareness and improving access to sensory information for people with end-stage RP.
Conflict of interest statement
Disclosure:
Figures


References
-
- Humayun MS, de Juan E Jr., Dagnelie G. The Bionic Eye: A Quarter Century of Retinal Prosthesis Research and Development. Ophthalmology .2016; 123: S89–S97. - PubMed
-
- Hartong DT, Berson EL, Dryja TP.. Retinitis pigmentosa. Lancet. 2006; 368: 1795–1809. - PubMed
-
- RetNet: Genes and Mapped Loci Causing Retinal Diseases. Available at: https://sph.uth.edu/retnet/disease.htm . Accessed January 24, 2021.
-
- Wang AL, Knight DK, Vu T-TT, et al. .. Retinitis Pigmentosa: Review of Current Treatment. Int Ophthalmol Clin .2019; 59: 263–280. - PubMed
-
- Dias MF, Joo K, Kemp JA, et al. .. Molecular genetics and emerging therapies for retinitis pigmentosa: basic research and clinical perspectives. Prog Retin Eye Res .2018; 63: 107–131. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

