Femtomolar SARS-CoV-2 Antigen Detection Using the Microbubbling Digital Assay with Smartphone Readout Enables Antigen Burden Quantitation and Tracking
- PMID: 34383886
- PMCID: PMC8436368
- DOI: 10.1093/clinchem/hvab158
Femtomolar SARS-CoV-2 Antigen Detection Using the Microbubbling Digital Assay with Smartphone Readout Enables Antigen Burden Quantitation and Tracking
Abstract
Background: High-sensitivity severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen assays are desirable to mitigate false negative results. Limited data are available to quantify and track SARS-CoV-2 antigen burden in respiratory samples from different populations.
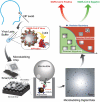
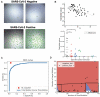
Methods: We developed the Microbubbling SARS-CoV-2 Antigen Assay (MSAA) with smartphone readout, with a limit of detection of 0.5 pg/mL (10.6 fmol/L) nucleocapsid antigen or 4000 copies/mL inactivated SARS-CoV-2 virus in nasopharyngeal (NP) swabs. We developed a computer vision and machine learning-based automatic microbubble image classifier to accurately identify positives and negatives and quantified and tracked antigen dynamics in intensive care unit coronavirus disease 2019 (COVID-19) inpatients and immunocompromised COVID-19 patients.
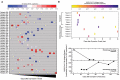
Results: Compared to qualitative reverse transcription-polymerase chain reaction methods, the MSAA demonstrated a positive percentage agreement of 97% (95% CI 92%-99%) and a negative percentage agreement of 97% (95% CI 94%-100%) in a clinical validation study with 372 residual clinical NP swabs. In immunocompetent individuals, the antigen positivity rate in swabs decreased as days-after-symptom-onset increased, despite persistent nucleic acid positivity. Antigen was detected for longer and variable periods of time in immunocompromised patients with hematologic malignancies. Total microbubble volume, a quantitative marker of antigen burden, correlated inversely with cycle threshold values and days-after-symptom-onset. Viral sequence variations were detected in patients with long duration of high antigen burden.
Conclusions: The MSAA enables sensitive and specific detection of acute infections and quantification and tracking of antigen burden and may serve as a screening method in longitudinal studies to identify patients who are likely experiencing active rounds of ongoing replication and warrant close viral sequence monitoring.
Keywords: SARS-CoV-2; longitudinal NP swab samples; microbubbling assay; viral antigen.
© American Association for Clinical Chemistry 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Update of
-
Femtomolar SARS-CoV-2 Antigen Detection Using the Microbubbling Digital Assay with Smartphone Readout Enables Antigen Burden Quantitation and Dynamics Tracking.medRxiv [Preprint]. 2021 Mar 26:2021.03.17.21253847. doi: 10.1101/2021.03.17.21253847. medRxiv. 2021. Update in: Clin Chem. 2021 Dec 30;68(1):230-239. doi: 10.1093/clinchem/hvab158. PMID: 33791710 Free PMC article. Updated. Preprint.
Similar articles
-
Femtomolar SARS-CoV-2 Antigen Detection Using the Microbubbling Digital Assay with Smartphone Readout Enables Antigen Burden Quantitation and Dynamics Tracking.medRxiv [Preprint]. 2021 Mar 26:2021.03.17.21253847. doi: 10.1101/2021.03.17.21253847. medRxiv. 2021. Update in: Clin Chem. 2021 Dec 30;68(1):230-239. doi: 10.1093/clinchem/hvab158. PMID: 33791710 Free PMC article. Updated. Preprint.
-
Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection.Clin Microbiol Infect. 2021 Feb;27(2):289.e1-289.e4. doi: 10.1016/j.cmi.2020.09.057. Epub 2020 Oct 5. Clin Microbiol Infect. 2021. PMID: 33031947 Free PMC article.
-
A Generic, Scalable, and Rapid Time-Resolved Förster Resonance Energy Transfer-Based Assay for Antigen Detection-SARS-CoV-2 as a Proof of Concept.mBio. 2021 May 18;12(3):e00902-21. doi: 10.1128/mBio.00902-21. mBio. 2021. PMID: 34006662 Free PMC article.
-
Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs.Biochem Med (Zagreb). 2021 Jun 15;31(2):020601. doi: 10.11613/BM.2021.020601. Biochem Med (Zagreb). 2021. PMID: 34140830 Free PMC article. Review.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
Cited by
-
Pretreatment Methods for Human Nasopharyngeal Swabs to Increase the Signal to Noise Ratio of High Sensitivity Immunoassays.ACS Meas Sci Au. 2022 Jun 29;2(5):414-421. doi: 10.1021/acsmeasuresciau.2c00024. eCollection 2022 Oct 19. ACS Meas Sci Au. 2022. PMID: 36785662 Free PMC article.
-
Debulking different Corona (SARS-CoV-2 delta, omicron, OC43) and Influenza (H1N1, H3N2) virus strains by plant viral trap proteins in chewing gums to decrease infection and transmission.Biomaterials. 2022 Sep;288:121671. doi: 10.1016/j.biomaterials.2022.121671. Epub 2022 Jul 18. Biomaterials. 2022. PMID: 35953331 Free PMC article. Clinical Trial.
-
Debulking SARS-CoV-2 in saliva using angiotensin converting enzyme 2 in chewing gum to decrease oral virus transmission and infection.Mol Ther. 2022 May 4;30(5):1966-1978. doi: 10.1016/j.ymthe.2021.11.008. Epub 2021 Nov 11. Mol Ther. 2022. PMID: 34774754 Free PMC article.
-
Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis.PLoS Med. 2021 Aug 12;18(8):e1003735. doi: 10.1371/journal.pmed.1003735. eCollection 2021 Aug. PLoS Med. 2021. PMID: 34383750 Free PMC article.
-
Dental Medicine and Engineering Unite to Transform Oral Health Innovations.J Dent Res. 2023 Oct;102(11):1177-1179. doi: 10.1177/00220345231183339. Epub 2023 Aug 7. J Dent Res. 2023. PMID: 37548396 Free PMC article.
References
-
- SARS-CoV-2 reference panel comparative data. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-dev... (Accessed June 2021).
-
- Abbasi J. Researchers tie severe immunosuppression to chronic COVID-19 and virus variants. JAMA 2021;325:2033–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

