Zfhx3 modulates retinal sensitivity and circadian responses to light
- PMID: 34383984
- PMCID: PMC9292409
- DOI: 10.1096/fj.202100563R
Zfhx3 modulates retinal sensitivity and circadian responses to light
Abstract
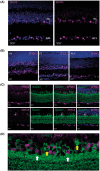
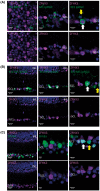
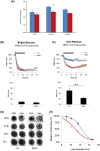
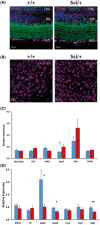
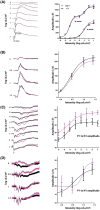
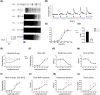
Mutations in transcription factors often exhibit pleiotropic effects related to their complex expression patterns and multiple regulatory targets. One such mutation in the zinc finger homeobox 3 (ZFHX3) transcription factor, short circuit (Sci, Zfhx3Sci/+ ), is associated with significant circadian deficits in mice. However, given evidence of its retinal expression, we set out to establish the effects of the mutation on retinal function using molecular, cellular, behavioral and electrophysiological measures. Immunohistochemistry confirms the expression of ZFHX3 in multiple retinal cell types, including GABAergic amacrine cells and retinal ganglion cells including intrinsically photosensitive retinal ganglion cells (ipRGCs). Zfhx3Sci/+ mutants display reduced light responsiveness in locomotor activity and circadian entrainment, relatively normal electroretinogram and optomotor responses but exhibit an unexpected pupillary reflex phenotype with markedly increased sensitivity. Furthermore, multiple electrode array recordings of Zfhx3Sci/+ retina show an increased sensitivity of ipRGC light responses.
Keywords: amacrine cell; light sensitivity; mutation; pleiotropy; pupillary reflex.
© 2021 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Villard J. Transcription regulation and human diseases. Swiss Med Wkly. 2004;134:571‐579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

