Comparing Smoking Cessation Interventions among Underserved Patients Referred for Lung Cancer Screening: A Pragmatic Trial Protocol
- PMID: 34384042
- PMCID: PMC8867367
- DOI: 10.1513/AnnalsATS.202104-499SD
Comparing Smoking Cessation Interventions among Underserved Patients Referred for Lung Cancer Screening: A Pragmatic Trial Protocol
Abstract
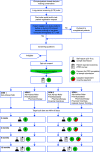
Smoking burdens are greatest among underserved patients. Lung cancer screening (LCS) reduces mortality among individuals at risk for smoking-associated lung cancer. Although LCS programs must offer smoking cessation support, the interventions that best promote cessation among underserved patients in this setting are unknown. This stakeholder-engaged, pragmatic randomized clinical trial will compare the effectiveness of four interventions promoting smoking cessation among underserved patients referred for LCS. By using an additive study design, all four arms provide standard "ask-advise-refer" care. Arm 2 adds free or subsidized pharmacologic cessation aids, arm 3 adds financial incentives up to $600 for cessation, and arm 4 adds a mobile device-delivered episodic future thinking tool to promote attention to long-term health goals. We hypothesize that smoking abstinence rates will be higher with the addition of each intervention when compared with arm 1. We will enroll 3,200 adults with LCS orders at four U.S. health systems. Eligible patients include those who smoke at least one cigarette daily and self-identify as a member of an underserved group (i.e., is Black or Latinx, is a rural resident, completed a high school education or less, and/or has a household income <200% of the federal poverty line). The primary outcome is biochemically confirmed smoking abstinence sustained through 6 months. Secondary outcomes include abstinence sustained through 12 months, other smoking-related clinical outcomes, and patient-reported outcomes. This pragmatic randomized clinical trial will identify the most effective smoking cessation strategies that LCS programs can implement to reduce smoking burdens affecting underserved populations. Clinical trial registered with clinicaltrials.gov (NCT04798664). Date of registration: March 12, 2021. Date of trial launch: May 17, 2021.
Keywords: lung cancer screening; pragmatic clinical trial; smoking cessation; tobacco cessation; vulnerable populations.
Figures


References
-
- Rostron BL, Chang CM, Pechacek TF. Estimation of cigarette smoking-attributable morbidity in the United States. JAMA Intern Med . 2014;174:1922–1928. - PubMed
-
- Moyer VA, U.S. Preventive Services Task Force Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med . 2014;160:330–338. - PubMed
-
- Kathuria H, Detterbeck FC, Fathi JT, Fennig K, Gould MK, Jolicoeur DG, et al. Stakeholder research priorities for smoking cessation interventions within lung cancer screening programs: an official American Thoracic Society research statement. Am J Respir Crit Care Med . 2017;196:1202–1212. - PMC - PubMed
-
- Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. U.S. Preventive Services Task Force Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA . 2021;325:962–970. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

