TPEN attenuates amyloid-β25-35-induced neuronal damage with changes in the electrophysiological properties of voltage-gated sodium and potassium channels
- PMID: 34384467
- PMCID: PMC8359616
- DOI: 10.1186/s13041-021-00837-z
TPEN attenuates amyloid-β25-35-induced neuronal damage with changes in the electrophysiological properties of voltage-gated sodium and potassium channels
Abstract
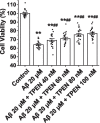
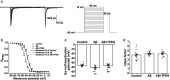
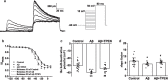
To understand the role of intracellular zinc ion (Zn2+) dysregulation in mediating age-related neurodegenerative changes, particularly neurotoxicity resulting from the generation of excessive neurotoxic amyloid-β (Aβ) peptides, this study aimed to investigate whether N, N, N', N'-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN), a Zn2+-specific chelator, could attenuate Aβ25-35-induced neurotoxicity and the underlying electrophysiological mechanism. We used the 3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay to measure the viability of hippocampal neurons and performed single-cell confocal imaging to detect the concentration of Zn2+ in these neurons. Furthermore, we used the whole-cell patch-clamp technique to detect the evoked repetitive action potential (APs), the voltage-gated sodium and potassium (K+) channels of primary hippocampal neurons. The analysis showed that TPEN attenuated Aβ25-35-induced neuronal death, reversed the Aβ25-35-induced increase in intracellular Zn2+ concentration and the frequency of APs, inhibited the increase in the maximum current density of voltage-activated sodium channel currents induced by Aβ25-35, relieved the Aβ25-35-induced decrease in the peak amplitude of transient outward K+ currents (IA) and outward-delayed rectifier K+ currents (IDR) at different membrane potentials, and suppressed the steady-state activation and inactivation curves of IA shifted toward the hyperpolarization direction caused by Aβ25-35. These results suggest that Aβ25-35-induced neuronal damage correlated with Zn2+ dysregulation mediated the electrophysiological changes in the voltage-gated sodium and K+ channels. Moreover, Zn2+-specific chelator-TPEN attenuated Aβ25-35-induced neuronal damage by recovering the intracellular Zn2+ concentration.
Keywords: Aβ25–35; Channel currents; TPEN; Voltage-gated potassium channels; Voltage-gated sodium channels; Zinc ions.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Role of TPEN in Amyloid-β25-35-Induced Neuronal Damage Correlating with Recovery of Intracellular Zn2+ and Intracellular Ca2+ Overloading.Mol Neurobiol. 2023 Aug;60(8):4232-4245. doi: 10.1007/s12035-023-03322-x. Epub 2023 Apr 14. Mol Neurobiol. 2023. PMID: 37059931
-
Genistein Inhibits Aβ25-35-Induced Neuronal Death with Changes in the Electrophysiological Properties of Voltage-Gated Sodium and Potassium Channels.Cell Mol Neurobiol. 2019 Aug;39(6):809-822. doi: 10.1007/s10571-019-00680-w. Epub 2019 Apr 30. Cell Mol Neurobiol. 2019. PMID: 31037516 Free PMC article.
-
Zn2+ reduction induces neuronal death with changes in voltage-gated potassium and sodium channel currents.J Trace Elem Med Biol. 2017 May;41:66-74. doi: 10.1016/j.jtemb.2017.02.011. Epub 2017 Feb 20. J Trace Elem Med Biol. 2017. PMID: 28347465
-
Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability.Pharmacol Ther. 2006 Sep;111(3):567-83. doi: 10.1016/j.pharmthera.2005.11.004. Epub 2006 Jan 10. Pharmacol Ther. 2006. PMID: 16410023 Review.
-
Voltage-Gated Potassium Channels: A Structural Examination of Selectivity and Gating.Cold Spring Harb Perspect Biol. 2016 May 2;8(5):a029231. doi: 10.1101/cshperspect.a029231. Cold Spring Harb Perspect Biol. 2016. PMID: 27141052 Free PMC article. Review.
Cited by
-
Role of TPEN in Amyloid-β25-35-Induced Neuronal Damage Correlating with Recovery of Intracellular Zn2+ and Intracellular Ca2+ Overloading.Mol Neurobiol. 2023 Aug;60(8):4232-4245. doi: 10.1007/s12035-023-03322-x. Epub 2023 Apr 14. Mol Neurobiol. 2023. PMID: 37059931
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

