Histone deacetylase inhibition leads to regulatory histone mark alterations and impairs meiosis in oocytes
- PMID: 34384478
- PMCID: PMC8359552
- DOI: 10.1186/s13072-021-00413-8
Histone deacetylase inhibition leads to regulatory histone mark alterations and impairs meiosis in oocytes
Abstract
Background: Panobinostat (PB), a histone deacetylase (HDAC) inhibitor drug, is clinically used in the treatment of cancers. We investigated the effects of PB on murine ovarian functions in embryos and adult animals.
Methods: C57BL/6J mice were treated with 5 mg/kg PB on alternate days from embryonic day (E) 6.5 to E15.5. We analysed the effects of PB on the ovaries by using immunofluorescence, gene expression analysis and DNA methylation analysis techniques.
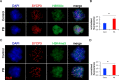
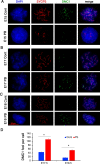
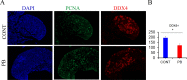
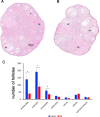
Results: At E15.5, we observed increases in histone H3K9Ac, H4Ac and H3K4me3 marks, while the level of the silencing H3K9me3 mark decreased. Synaptonemal complex examination at E15.5, E17.5 and E18.5 showed a delay in meiotic progression characterized by the absence of synaptonemal complexes at E15.5 and the persistence of double-strand breaks (DSBs) at E17.5 and E18.5 in PB-exposed oocytes. We found that exposure to PB led to changes in the expression of 1169 transcripts at E15.5. Genes regulated by the male-specific factors SRY-Box Transcription Factor 9 (SOX9) and Doublesex and Mab-3-related Transcription factor 1 (DMRT1) were among the most upregulated genes in the ovaries of PB-exposed mice. In contrast, PB treatment led to decreases in the expression of genes regulated by the WNT4 pathway. Notably, we observed 119 deregulated genes encoding Zn-finger proteins. The observed alterations in epigenetic marks and gene expression correlated with decreases in the numbers of germ cells at E15.5. After birth, PB-exposed ovaries showed increased proliferation of primary and secondary follicles. We also observed decreases in the numbers of primordial, primary and secondary follicles in adult ovaries from mice that were exposed to PB in utero. Finally, epigenetic alterations such as decreased H3K4me3 and increased H4 acetylation levels were also detected in somatic cells surrounding fully grown oocytes.
Conclusion: Our data suggest that inhibition of histone deacetylase by PB during a critical developmental window affects reprogramming and germ cell specification via alteration of epigenetic marks.
Keywords: Epigenetics; HDAC; Histone modifications; Ovary; Panobinostat; RNA-seq.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

