Effects of antibiotic resistance, drug target attainment, bacterial pathogenicity and virulence, and antibiotic access and affordability on outcomes in neonatal sepsis: an international microbiology and drug evaluation prospective substudy (BARNARDS)
- PMID: 34384533
- PMCID: PMC8612937
- DOI: 10.1016/S1473-3099(21)00050-5
Effects of antibiotic resistance, drug target attainment, bacterial pathogenicity and virulence, and antibiotic access and affordability on outcomes in neonatal sepsis: an international microbiology and drug evaluation prospective substudy (BARNARDS)
Abstract
Background: Sepsis is a major contributor to neonatal mortality, particularly in low-income and middle-income countries (LMICs). WHO advocates ampicillin-gentamicin as first-line therapy for the management of neonatal sepsis. In the BARNARDS observational cohort study of neonatal sepsis and antimicrobial resistance in LMICs, common sepsis pathogens were characterised via whole genome sequencing (WGS) and antimicrobial resistance profiles. In this substudy of BARNARDS, we aimed to assess the use and efficacy of empirical antibiotic therapies commonly used in LMICs for neonatal sepsis.
Methods: In BARNARDS, consenting mother-neonates aged 0-60 days dyads were enrolled on delivery or neonatal presentation with suspected sepsis at 12 BARNARDS clinical sites in Bangladesh, Ethiopia, India, Pakistan, Nigeria, Rwanda, and South Africa. Stillborn babies were excluded from the study. Blood samples were collected from neonates presenting with clinical signs of sepsis, and WGS and minimum inhibitory concentrations for antibiotic treatment were determined for bacterial isolates from culture-confirmed sepsis. Neonatal outcome data were collected following enrolment until 60 days of life. Antibiotic usage and neonatal outcome data were assessed. Survival analyses were adjusted to take into account potential clinical confounding variables related to the birth and pathogen. Additionally, resistance profiles, pharmacokinetic-pharmacodynamic probability of target attainment, and frequency of resistance (ie, resistance defined by in-vitro growth of isolates when challenged by antibiotics) were assessed. Questionnaires on health structures and antibiotic costs evaluated accessibility and affordability.
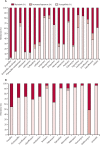
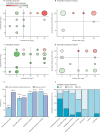
Findings: Between Nov 12, 2015, and Feb 1, 2018, 36 285 neonates were enrolled into the main BARNARDS study, of whom 9874 had clinically diagnosed sepsis and 5749 had available antibiotic data. The four most commonly prescribed antibiotic combinations given to 4451 neonates (77·42%) of 5749 were ampicillin-gentamicin, ceftazidime-amikacin, piperacillin-tazobactam-amikacin, and amoxicillin clavulanate-amikacin. This dataset assessed 476 prescriptions for 442 neonates treated with one of these antibiotic combinations with WGS data (all BARNARDS countries were represented in this subset except India). Multiple pathogens were isolated, totalling 457 isolates. Reported mortality was lower for neonates treated with ceftazidime-amikacin than for neonates treated with ampicillin-gentamicin (hazard ratio [adjusted for clinical variables considered potential confounders to outcomes] 0·32, 95% CI 0·14-0·72; p=0·0060). Of 390 Gram-negative isolates, 379 (97·2%) were resistant to ampicillin and 274 (70·3%) were resistant to gentamicin. Susceptibility of Gram-negative isolates to at least one antibiotic in a treatment combination was noted in 111 (28·5%) to ampicillin-gentamicin; 286 (73·3%) to amoxicillin clavulanate-amikacin; 301 (77·2%) to ceftazidime-amikacin; and 312 (80·0%) to piperacillin-tazobactam-amikacin. A probability of target attainment of 80% or more was noted in 26 neonates (33·7% [SD 0·59]) of 78 with ampicillin-gentamicin; 15 (68·0% [3·84]) of 27 with amoxicillin clavulanate-amikacin; 93 (92·7% [0·24]) of 109 with ceftazidime-amikacin; and 70 (85·3% [0·47]) of 76 with piperacillin-tazobactam-amikacin. However, antibiotic and country effects could not be distinguished. Frequency of resistance was recorded most frequently with fosfomycin (in 78 isolates [68·4%] of 114), followed by colistin (55 isolates [57·3%] of 96), and gentamicin (62 isolates [53·0%] of 117). Sites in six of the seven countries (excluding South Africa) stated that the cost of antibiotics would influence treatment of neonatal sepsis.
Interpretation: Our data raise questions about the empirical use of combined ampicillin-gentamicin for neonatal sepsis in LMICs because of its high resistance and high rates of frequency of resistance and low probability of target attainment. Accessibility and affordability need to be considered when advocating antibiotic treatments with variance in economic health structures across LMICs.
Funding: The Bill & Melinda Gates Foundation.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures



Comment in
-
Antibiotics for neonatal sepsis in low-income and middle-income countries-where to go from here?Lancet Infect Dis. 2021 Dec;21(12):1617-1618. doi: 10.1016/S1473-3099(21)00199-7. Epub 2021 Aug 9. Lancet Infect Dis. 2021. PMID: 34384534 No abstract available.
References
-
- UN Inter-agency Group for Child Mortality Estimation . UN Inter-agency Group for Child Mortality Estimation; New York: 2018. Mortality estimation, 2018. Levels and trends in child mortality 2018.
-
- Puopolo KM, Benitz WE, Zaoutis TE. Management of neonates born at ≥35 0/7 weeks gestation with suspected or proven early onset bacterial sepsis. Pediatrics. 2018;142 - PubMed
-
- Puopolo KM, Benitz WE, Zaoutis TE. Management of neonates born at ≤34 6/7 weeks gestation with suspected or proven early onset bacterial sepsis. Pediatrics. 2018;142 - PubMed
-
- The World Bank Mortality rate, infant (per 1000 live births) https://data.worldbank.org/indicator/SP.DYN.IMRT.IN
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

