Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE
- PMID: 34384544
- PMCID: PMC8380737
- DOI: 10.1016/j.cell.2021.07.021
Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE
Abstract
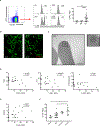
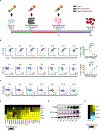
Emerging evidence supports that mitochondrial dysfunction contributes to systemic lupus erythematosus (SLE) pathogenesis. Here we show that programmed mitochondrial removal, a hallmark of mammalian erythropoiesis, is defective in SLE. Specifically, we demonstrate that during human erythroid cell maturation, a hypoxia-inducible factor (HIF)-mediated metabolic switch is responsible for the activation of the ubiquitin-proteasome system (UPS), which precedes and is necessary for the autophagic removal of mitochondria. A defect in this pathway leads to accumulation of red blood cells (RBCs) carrying mitochondria (Mito+ RBCs) in SLE patients and in correlation with disease activity. Antibody-mediated internalization of Mito+ RBCs induces type I interferon (IFN) production through activation of cGAS in macrophages. Accordingly, SLE patients carrying both Mito+ RBCs and opsonizing antibodies display the highest levels of blood IFN-stimulated gene (ISG) signatures, a distinctive feature of SLE.
Keywords: CANDLE syndrome; HIF2a; autoimmunity; cGAS; human erythropoiesis; interferon; mitochondrial DNA; mitophagy; proteasome; systemic lupus erythematosus.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests V.P. has received consulting honoraria from Sanofi, Astra Zeneca, and Moderna and is the recipient of a research grant from Sanofi and a contract from Astra Zeneca. J.F.B. is a member of the S.A.B. of Neovacs.
Figures




Comment in
-
Erythrocyte-derived mitochondria take to the lupus stage.Cell Metab. 2021 Sep 7;33(9):1723-1725. doi: 10.1016/j.cmet.2021.08.008. Cell Metab. 2021. PMID: 34496229
-
RBC mitochondria trigger interferon release.Nat Rev Nephrol. 2021 Nov;17(11):707. doi: 10.1038/s41581-021-00494-4. Nat Rev Nephrol. 2021. PMID: 34545228 No abstract available.
-
Mitochondrial dysfunction in the erythroid compartment.Nat Immunol. 2021 Nov;22(11):1354-1355. doi: 10.1038/s41590-021-01050-9. Nat Immunol. 2021. PMID: 34671144 Free PMC article.
References
-
- Ahlqvist KJ, Leoncini S, Pecorelli A, Wortmann SB, Ahola S, Forsstrom S, Guerranti R, De Felice C, Smeitink J, Ciccoli L, et al. (2015). MtDNA mutagenesis impairs elimination of mitochondria during erythroid maturation leading to enhanced erythrocyte destruction. Nat Commun 6, 6494. - PubMed
-
- Ait-Ali N, Fridlich R, Millet-Puel G, Clerin E, Delalande F, Jaillard C, Blond F, Perrocheau L, Reichman S, Byrne LC, et al. (2015). Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 161, 817–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

