Stevens-Johnson syndrome post second dose of Pfizer COVID-19 vaccine: a case report
- PMID: 34384729
- PMCID: PMC8288232
- DOI: 10.1016/j.oooo.2021.06.019
Stevens-Johnson syndrome post second dose of Pfizer COVID-19 vaccine: a case report
Abstract
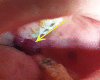
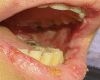
Coronavirus disease 2019 (COVID-19) began in December 2019 and has affected millions of people all over the world. Respiratory illness in the form of severe pneumonia, in addition to multiorgan failure and death, is the clinical spectrum of COVID-19. Although there are no specific therapeutic agents for COVID-19 infection, the COVID-19 vaccine reduces morbidity and mortality associated with COVID-19 infection and is generally well tolerated. We report one potential complication of the Pfizer COVID-19 vaccine: a known case of Stevens-Johnson syndrome (SJS) that occurred after the second dose of the Pfizer COVID-19 vaccine alone without exposure to any other drug. Despite the initial severe adverse reaction, the patient showed a full recovery. Although SJS can be associated with COVID-19 vaccination, it is rare, and the benefits of receiving the vaccination outweigh the potential harms.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
References
-
- Łoboda J, Dudzik A, Chomyszyn-Gajewska M. Stevens-Johnson syndrom and toxic epidermal necrolysis–based on literature. Przegl Lek. 2015;72:35–37. - PubMed
-
- Tristani-Firouzi P, Petersena MJ, Saffle JR, Morris SE, Zone JJ. Treatment of toxic epidermal necrolysis with intravenous immunoglobulin in children. J Am Acad Dermatol. 2002;47:548–552. - PubMed
-
- Prins C, Kerdel FA, Padilla S, et al. Treatment of toxic epidermal necrolysis with high-dose intravenous immunoglobulins: multicenter retrospective analysis of 48 consecutive cases. Arch Dermatol. 2003;139:26–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical