Mechanosensitive axon outgrowth mediated by L1-laminin clutch interface
- PMID: 34384760
- PMCID: PMC8456307
- DOI: 10.1016/j.bpj.2021.08.009
Mechanosensitive axon outgrowth mediated by L1-laminin clutch interface
Abstract
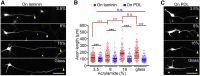
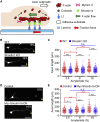
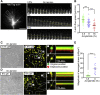
Mechanical properties of the extracellular environment modulate axon outgrowth. Growth cones at the tip of extending axons generate traction force for axon outgrowth by transmitting the force of actin filament retrograde flow, produced by actomyosin contraction and F-actin polymerization, to adhesive substrates through clutch and cell adhesion molecules. A molecular clutch between the actin filament flow and substrate is proposed to contribute to cellular mechanosensing. However, the molecular identity of the clutch interface responsible for mechanosensitive growth cone advance is unknown. We previously reported that mechanical coupling between actin filament retrograde flow and adhesive substrates through the clutch molecule shootin1a and the cell adhesion molecule L1 generates traction force for axon outgrowth and guidance. Here, we show that cultured mouse hippocampal neurons extend longer axons on stiffer substrates under elastic conditions that correspond to the soft brain environments. We demonstrate that this stiffness-dependent axon outgrowth requires actin-adhesion coupling mediated by shootin1a, L1, and laminin on the substrate. Speckle imaging analyses showed that L1 at the growth cone membrane switches between two adhesive states: L1 that is immobilized and that undergoes retrograde movement on the substrate. The duration of the immobilized phase was longer on stiffer substrates; this was accompanied by increases in actin-adhesion coupling and in the traction force exerted on the substrate. These data suggest that the interaction between L1 and laminin is enhanced on stiffer substrates, thereby promoting force generation for axon outgrowth.
Copyright © 2021 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Analyses of Actin Dynamics, Clutch Coupling and Traction Force for Growth Cone Advance.J Vis Exp. 2021 Oct 21;(176). doi: 10.3791/63227. J Vis Exp. 2021. PMID: 34747402
-
Guidance of Axons by Local Coupling of Retrograde Flow to Point Contact Adhesions.J Neurosci. 2016 Feb 17;36(7):2267-82. doi: 10.1523/JNEUROSCI.2645-15.2016. J Neurosci. 2016. PMID: 26888936 Free PMC article.
-
Adhesion-clutch between DCC and netrin-1 mediates netrin-1-induced axonal haptotaxis.Front Mol Neurosci. 2024 Feb 5;17:1307755. doi: 10.3389/fnmol.2024.1307755. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38375502 Free PMC article.
-
Role of myosin II in axon outgrowth.J Histochem Cytochem. 2003 Apr;51(4):421-8. doi: 10.1177/002215540305100403. J Histochem Cytochem. 2003. PMID: 12642620 Review.
-
Actin dynamics in growth cone motility and navigation.J Neurochem. 2014 Apr;129(2):221-34. doi: 10.1111/jnc.12506. Epub 2013 Nov 17. J Neurochem. 2014. PMID: 24164353 Free PMC article. Review.
Cited by
-
Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets.Signal Transduct Target Ther. 2023 Jul 31;8(1):282. doi: 10.1038/s41392-023-01501-9. Signal Transduct Target Ther. 2023. PMID: 37518181 Free PMC article. Review.
-
Mice Mutated in the First Fibronectin Domain of Adhesion Molecule L1 Show Brain Malformations and Behavioral Abnormalities.Biomolecules. 2024 Apr 11;14(4):468. doi: 10.3390/biom14040468. Biomolecules. 2024. PMID: 38672483 Free PMC article.
-
Basement membranes are crucial for proper olfactory placode shape, position and boundary with the brain, and for olfactory axon development.Elife. 2024 Dec 23;12:RP92004. doi: 10.7554/eLife.92004. Elife. 2024. PMID: 39713923 Free PMC article.
-
Interrogating the Molecular Clutch in Neuronal Growth Cones: Measuring Traction Forces, F-actin Retrograde Flow, and Point Contact Demographics.Methods Mol Biol. 2024;2831:251-264. doi: 10.1007/978-1-0716-3969-6_17. Methods Mol Biol. 2024. PMID: 39134855
-
Rlip76: An Unexplored Player in Neurodegeneration and Alzheimer's Disease?Int J Mol Sci. 2022 May 29;23(11):6098. doi: 10.3390/ijms23116098. Int J Mol Sci. 2022. PMID: 35682775 Free PMC article. Review.
References
-
- Franze K. Integrating chemistry and mechanics: the forces driving axon growth. Annu. Rev. Cell Dev. Biol. 2020;36:61–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

