SARS-CoV-2 detection by reverse transcriptase polymerase chain reaction testing: Analysis of false positive results and recommendations for quality control measures
- PMID: 34385110
- PMCID: PMC8336987
- DOI: 10.1016/j.prp.2021.153579
SARS-CoV-2 detection by reverse transcriptase polymerase chain reaction testing: Analysis of false positive results and recommendations for quality control measures
Abstract
Testing for SARS-CoV-2 has become a critical component for the management of the COVID-19 pandemic. Reverse transcriptase polymerase chain reaction (RT-PCR) assays are currently the predominate method for testing. Quality control (QC) measures utilize known positive and known negative controls to ensure the adequacy of extraction and RT-PCR steps but do not evaluate all components of testing. We have conducted a quality assurance review of our RT-PCR testing for COVID-19 to determine the rate of false positive results in asymptomatic patients and causes for these errors.
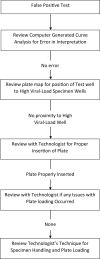
Design: We have developed a quality control procedure in which all specimens from asymptomatic unexposed persons with SARS-CoV-2 positive tests were retested. When a second test was "non-detected" a third test was performed and a root cause analysis of the erroneous result undertaken.
Results: In the study period, 24,717 samples were tested and 6251 were from asymptomatic patients. Of the 288 initial positive tests, 20 (6.9%) were negative on retesting. Review of cycle threshold curves, technologists' records, location of specimen on testing plates and relationships with high viral load specimens was undertaken. Analysis revealed technologists' errors (misplacement of specimen in testing plate or contamination) and cross contamination from high viral load specimens in adjacent wells of testing plates were common causes for false positive results.
Discussion: SARS-CoV-2 RT-PCR testing is associated with a small number of false positive results, most easily recognized in asymptomatic non-exposed patients. Implementation of a limited retesting protocol identifies clinically significant testing errors and allows review and improvement of laboratory procedures.
Keywords: COVID-19; False positive results; Polymerase chain reaction; Quality control; SARS-CoV-2.
Copyright © 2021 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures
Similar articles
-
Discrimination of False Negative Results in RT-PCR Detection of SARS-CoV-2 RNAs in Clinical Specimens by Using an Internal Reference.Virol Sin. 2020 Dec;35(6):758-767. doi: 10.1007/s12250-020-00273-8. Epub 2020 Aug 4. Virol Sin. 2020. PMID: 32749593 Free PMC article.
-
Evaluation of the INDICAID COVID-19 Rapid Antigen Test in Symptomatic Populations and Asymptomatic Community Testing.Microbiol Spectr. 2021 Sep 3;9(1):e0034221. doi: 10.1128/Spectrum.00342-21. Epub 2021 Aug 4. Microbiol Spectr. 2021. PMID: 34346748 Free PMC article.
-
Impact of coronavirus disease 2019 (COVID-19) pre-test probability on positive predictive value of high cycle threshold severe acute respiratory coronavirus virus 2 (SARS-CoV-2) real-time reverse transcription polymerase chain reaction (RT-PCR) test results.Infect Control Hosp Epidemiol. 2022 Sep;43(9):1179-1183. doi: 10.1017/ice.2021.369. Epub 2021 Aug 9. Infect Control Hosp Epidemiol. 2022. PMID: 34369325 Free PMC article.
-
A systematic review of the sensitivity and specificity of lateral flow devices in the detection of SARS-CoV-2.BMC Infect Dis. 2021 Aug 18;21(1):828. doi: 10.1186/s12879-021-06528-3. BMC Infect Dis. 2021. PMID: 34407759 Free PMC article.
-
Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance.Sci Total Environ. 2022 Jan 20;805:149877. doi: 10.1016/j.scitotenv.2021.149877. Epub 2021 Aug 25. Sci Total Environ. 2022. PMID: 34818780 Free PMC article. Review.
Cited by
-
Advanced Plasmonic Nanoparticle-Based Techniques for the Prevention, Detection, and Treatment of Current COVID-19.Plasmonics. 2023;18(1):311-347. doi: 10.1007/s11468-022-01754-0. Epub 2022 Dec 23. Plasmonics. 2023. PMID: 36588744 Free PMC article. Review.
-
Strategies to Overcome Erroneous Outcomes in Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Testing: Insights From the COVID-19 Pandemic.Cureus. 2024 Nov 3;16(11):e72954. doi: 10.7759/cureus.72954. eCollection 2024 Nov. Cureus. 2024. PMID: 39498425 Free PMC article. Review.
-
UK ethnic minority healthcare workers' perspectives on COVID-19 vaccine hesitancy in the UK ethnic minority community: A qualitative study.Front Psychol. 2022 Aug 3;13:908917. doi: 10.3389/fpsyg.2022.908917. eCollection 2022. Front Psychol. 2022. PMID: 35992396 Free PMC article.
-
Evaluation of a fluorescence in situ hybridization (FISH)-based method for detection of SARS-CoV-2 in saliva.PLoS One. 2022 Nov 8;17(11):e0277367. doi: 10.1371/journal.pone.0277367. eCollection 2022. PLoS One. 2022. PMID: 36346813 Free PMC article.
-
Real-Time Polymerase Chain Reaction: Current Techniques, Applications, and Role in COVID-19 Diagnosis.Genes (Basel). 2022 Dec 16;13(12):2387. doi: 10.3390/genes13122387. Genes (Basel). 2022. PMID: 36553654 Free PMC article. Review.
References
-
- L.J. Layfield, S. Camp, K. Bowers, D.C. Miller, Quality control practice for SARS-CoV-2 PCR nucleic acid detection: an additional quality control measure, in: Proceedings of the USCAP Annual Meeting, March 2021.
-
- Woloshin S., Patel N., Kesselheim A.S. Perspective: false negative tests for SARS-CoV-2 infection – challenges and implications. N. Engl. J. Med. 2020;383 - PubMed
-
- Arevalo-Rodriguez I., Buitrago-Garcia D., Simancas-Racines D., Zambrano-Achig P., Del Campo R., Ciapponi A., Sued O., Martinez-García L., Rutjes A.W., Low N., Bossuyt P.M., Perez-Molina J.A., Zamora J. False negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12) - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous