Stem Cell Factor SOX2 Confers Ferroptosis Resistance in Lung Cancer via Upregulation of SLC7A11
- PMID: 34385181
- PMCID: PMC8530936
- DOI: 10.1158/0008-5472.CAN-21-0567
Stem Cell Factor SOX2 Confers Ferroptosis Resistance in Lung Cancer via Upregulation of SLC7A11
Abstract
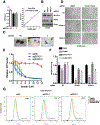
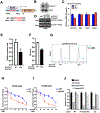
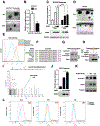
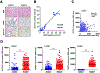
Ferroptosis is a lipid peroxidation-dependent cell death caused by metabolic dysfunction. Ferroptosis-associated enzymes are promising therapeutic targets for cancer treatment. However, such therapeutic strategies show limited efficacy due to drug resistance and other largely unknown underlying mechanisms. Here we report that cystine transporter SLC7A11 is upregulated in lung cancer stem-like cells (CSLC) and can be activated by stem cell transcriptional factor SOX2. Mutation of SOX2 binding site in SLC7A11 promoter reduced SLC7A11 expression and increased sensitivity to ferroptosis in cancer cells. Oxidation at Cys265 of SOX2 inhibited its activity and decreased the self-renewal capacity of CSLCs. Moreover, tumors with high SOX2 expression were more resistant to ferroptosis, and SLC7A11 expression was positively correlated with SOX2 in both mouse and human lung cancer tissue. Together, our study provides a mechanism by which cancer cells evade ferroptosis and suggests that oxidation of SOX2 can be a potential therapeutic target for cancer treatment. SIGNIFICANCE: This study uncovers a SOX2-SLC7A11 regulatory axis that confers resistance to ferroptosis in lung cancer stem-like cells.
©2021 American Association for Cancer Research.
Conflict of interest statement
CONFLICTS OF INTEREST STATEMENT:
The authors declare no potential conflicts of interest.
Figures



References
-
- Zheng J and Conrad M, The Metabolic Underpinnings of Ferroptosis. Cell Metab, 2020. 32(6): p. 920–937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

