HMGB1 released from nociceptors mediates inflammation
- PMID: 34385304
- PMCID: PMC8379951
- DOI: 10.1073/pnas.2102034118
HMGB1 released from nociceptors mediates inflammation
Abstract
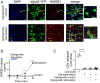
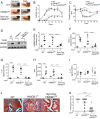
Inflammation, the body's primary defensive response system to injury and infection, is triggered by molecular signatures of microbes and tissue injury. These molecules also stimulate specialized sensory neurons, termed nociceptors. Activation of nociceptors mediates inflammation through antidromic release of neuropeptides into infected or injured tissue, producing neurogenic inflammation. Because HMGB1 is an important inflammatory mediator that is synthesized by neurons, we reasoned nociceptor release of HMGB1 might be a component of the neuroinflammatory response. In support of this possibility, we show here that transgenic nociceptors expressing channelrhodopsin-2 (ChR2) directly release HMGB1 in response to light stimulation. Additionally, HMGB1 expression in neurons was silenced by crossing synapsin-Cre (Syn-Cre) mice with floxed HMGB1 mice (HMGB1f/f). When these mice undergo sciatic nerve injury to activate neurogenic inflammation, they are protected from the development of cutaneous inflammation and allodynia as compared to wild-type controls. Syn-Cre/HMGB1fl/fl mice subjected to experimental collagen antibody-induced arthritis, a disease model in which nociceptor-dependent inflammation plays a significant pathological role, are protected from the development of allodynia and joint inflammation. Thus, nociceptor HMGB1 is required to mediate pain and inflammation during sciatic nerve injury and collagen antibody-induced arthritis.
Keywords: DAMP; HMGB1; arthritis; cytokine; sciatic nerve injury.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Inhibitor kappa B kinase beta dependent cytokine upregulation in nociceptive neurons contributes to nociceptive hypersensitivity after sciatic nerve injury.J Pain. 2012 May;13(5):485-97. doi: 10.1016/j.jpain.2012.02.010. J Pain. 2012. PMID: 22564672
-
Epineural optogenetic activation of nociceptors initiates and amplifies inflammation.Nat Biotechnol. 2021 Feb;39(2):179-185. doi: 10.1038/s41587-020-0673-2. Epub 2020 Sep 21. Nat Biotechnol. 2021. PMID: 32958958 Free PMC article.
-
Optogenetic Silencing of Nav1.8-Positive Afferents Alleviates Inflammatory and Neuropathic Pain.eNeuro. 2016 Mar 16;3(1):ENEURO.0140-15.2016. doi: 10.1523/ENEURO.0140-15.2016. eCollection 2016 Jan-Feb. eNeuro. 2016. PMID: 27022626 Free PMC article.
-
Neurons Are a Primary Driver of Inflammation via Release of HMGB1.Cells. 2021 Oct 18;10(10):2791. doi: 10.3390/cells10102791. Cells. 2021. PMID: 34685772 Free PMC article. Review.
-
Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons.Curr Opin Neurobiol. 2020 Jun;62:17-25. doi: 10.1016/j.conb.2019.11.006. Epub 2019 Dec 3. Curr Opin Neurobiol. 2020. PMID: 31809997 Free PMC article. Review.
Cited by
-
Nonresolving inflammation redux.Immunity. 2022 Apr 12;55(4):592-605. doi: 10.1016/j.immuni.2022.03.016. Immunity. 2022. PMID: 35417674 Free PMC article. Review.
-
IGF2BP3 prevent HMGB1 mRNA decay in bladder cancer and development.Cell Mol Biol Lett. 2024 Mar 19;29(1):39. doi: 10.1186/s11658-024-00545-1. Cell Mol Biol Lett. 2024. PMID: 38504159 Free PMC article.
-
The Importance of Phosphoinositide 3-Kinase in Neuroinflammation.Int J Mol Sci. 2024 Oct 30;25(21):11638. doi: 10.3390/ijms252111638. Int J Mol Sci. 2024. PMID: 39519189 Free PMC article. Review.
-
High Mobility Group Box 1 (HMGB1): Molecular Signaling and Potential Therapeutic Strategies.Cells. 2024 Nov 23;13(23):1946. doi: 10.3390/cells13231946. Cells. 2024. PMID: 39682695 Free PMC article. Review.
-
HMGB1 is a critical molecule in the pathogenesis of Gram-negative sepsis.J Intensive Med. 2022 Mar 9;2(3):156-166. doi: 10.1016/j.jointm.2022.02.001. eCollection 2022 Jul. J Intensive Med. 2022. PMID: 36789020 Free PMC article. Review.
References
-
- Burke R. E., Sir Charles Sherrington’s the integrative action of the nervous system: a centenary appreciation. Brain 130, 887–894 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

