CO2 enhances the formation, nutrient scavenging and drug resistance properties of C. albicans biofilms
- PMID: 34385462
- PMCID: PMC8361082
- DOI: 10.1038/s41522-021-00238-z
CO2 enhances the formation, nutrient scavenging and drug resistance properties of C. albicans biofilms
Abstract
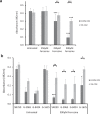
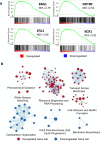
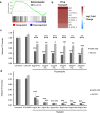
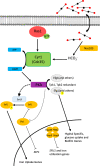
C. albicans is the predominant human fungal pathogen and frequently colonises medical devices, such as voice prostheses, as a biofilm. It is a dimorphic yeast that can switch between yeast and hyphal forms in response to environmental cues, a property that is essential during biofilm establishment and maturation. One such cue is the elevation of CO2 levels, as observed in exhaled breath for example. However, despite the clear medical relevance, the effect of CO2 on C. albicans biofilm growth has not been investigated to date. Here we show that physiologically relevant CO2 elevation enhances each stage of the C. albicans biofilm-forming process: from attachment through maturation to dispersion. The effects of CO2 are mediated via the Ras/cAMP/PKA signalling pathway and the central biofilm regulators Efg1, Brg1, Bcr1 and Ndt80. Biofilms grown under elevated CO2 conditions also exhibit increased azole resistance, increased Sef1-dependent iron scavenging and enhanced glucose uptake to support their rapid growth. These findings suggest that C. albicans has evolved to utilise the CO2 signal to promote biofilm formation within the host. We investigate the possibility of targeting CO2-activated processes and propose 2-deoxyglucose as a drug that may be repurposed to prevent C. albicans biofilm formation on medical airway management implants. We thus characterise the mechanisms by which CO2 promotes C. albicans biofilm formation and suggest new approaches for future preventative strategies.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Genetic control of conventional and pheromone-stimulated biofilm formation in Candida albicans.PLoS Pathog. 2013;9(4):e1003305. doi: 10.1371/journal.ppat.1003305. Epub 2013 Apr 18. PLoS Pathog. 2013. PMID: 23637598 Free PMC article.
-
Iron deprivation induces EFG1-mediated hyphal development in Candida albicans without affecting biofilm formation.FEMS Yeast Res. 2008 Aug;8(5):744-55. doi: 10.1111/j.1567-1364.2008.00394.x. Epub 2008 Jun 10. FEMS Yeast Res. 2008. PMID: 18547332
-
S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms.Virulence. 2017 Nov 17;8(8):1602-1617. doi: 10.1080/21505594.2017.1326438. Epub 2017 Jun 1. Virulence. 2017. PMID: 28481721 Free PMC article.
-
Molecular Determinants Involved in Candida albicans Biofilm Formation and Regulation.Mol Biotechnol. 2024 Jul;66(7):1640-1659. doi: 10.1007/s12033-023-00796-x. Epub 2023 Jul 6. Mol Biotechnol. 2024. PMID: 37410258 Review.
-
[Genetic regulatory mechanisms of Candida albicans biofilm formation].Sheng Wu Gong Cheng Xue Bao. 2017 Sep 25;33(9):1567-1581. doi: 10.13345/j.cjb.170122. Sheng Wu Gong Cheng Xue Bao. 2017. PMID: 28956402 Review. Chinese.
Cited by
-
The Pga59 cell wall protein is an amyloid forming protein involved in adhesion and biofilm establishment in the pathogenic yeast Candida albicans.NPJ Biofilms Microbiomes. 2023 Jan 25;9(1):6. doi: 10.1038/s41522-023-00371-x. NPJ Biofilms Microbiomes. 2023. PMID: 36697414 Free PMC article.
-
Accidental and Regulated Cell Death in Yeast Colony Biofilms.Bull Math Biol. 2025 Jul 17;87(8):110. doi: 10.1007/s11538-025-01470-w. Bull Math Biol. 2025. PMID: 40673959 Free PMC article.
-
Stress Conditions Affect the Immunomodulatory Potential of Candida albicans Extracellular Vesicles and Their Impact on Cytokine Release by THP-1 Human Macrophages.Int J Mol Sci. 2023 Dec 6;24(24):17179. doi: 10.3390/ijms242417179. Int J Mol Sci. 2023. PMID: 38139005 Free PMC article.
-
A CO2 sensing module modulates β-1,3-glucan exposure in Candida albicans.mBio. 2024 Feb 14;15(2):e0189823. doi: 10.1128/mbio.01898-23. Epub 2024 Jan 23. mBio. 2024. PMID: 38259065 Free PMC article.
-
Cell dispersion during biofilm formation by Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum and Lomentospora prolificans.Curr Res Microb Sci. 2023 May 10;4:100191. doi: 10.1016/j.crmicr.2023.100191. eCollection 2023. Curr Res Microb Sci. 2023. PMID: 37229517 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

