Next-generation cancer organoids
- PMID: 34385685
- PMCID: PMC12276900
- DOI: 10.1038/s41563-021-01057-5
Next-generation cancer organoids
Abstract
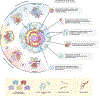
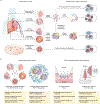
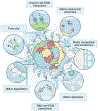
Organotypic models of patient-specific tumours are revolutionizing our understanding of cancer heterogeneity and its implications for personalized medicine. These advancements are, in part, attributed to the ability of organoid models to stably preserve genetic, proteomic, morphological and pharmacotypic features of the parent tumour in vitro, while also offering unprecedented genomic and environmental manipulation. Despite recent innovations in organoid protocols, current techniques for cancer organoid culture are inherently uncontrolled and irreproducible, owing to several non-standardized facets including cancer tissue sources and subsequent processing, medium formulations, and animal-derived three-dimensional matrices. Given the potential for cancer organoids to accurately recapitulate the intra- and intertumoral biological heterogeneity associated with patient-specific cancers, eliminating the undesirable technical variability accompanying cancer organoid culture is necessary to establish reproducible platforms that accelerate translatable insights into patient care. Here we describe the current challenges and recent multidisciplinary advancements and opportunities for standardizing next-generation cancer organoid systems.
© 2021. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
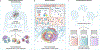

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

