Control of the activity of CAR-T cells within tumours via focused ultrasound
- PMID: 34385696
- PMCID: PMC9015817
- DOI: 10.1038/s41551-021-00779-w
Control of the activity of CAR-T cells within tumours via focused ultrasound
Abstract
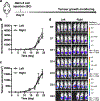
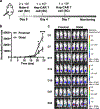
Focused ultrasound can deliver energy safely and non-invasively into tissues at depths of centimetres. Here we show that the genetics and cellular functions of chimeric antigen receptor T cells (CAR-T cells) within tumours can be reversibly controlled by the heat generated by short pulses of focused ultrasound via a CAR cassette under the control of a promoter for the heat-shock protein. In mice with subcutaneous tumours, locally injected T cells with the inducible CAR and activated via focused ultrasound guided by magnetic resonance imaging mitigated on-target off-tumour activity and enhanced the suppression of tumour growth, compared with the performance of non-inducible CAR-T cells. Acoustogenetic control of the activation of engineered T cells may facilitate the design of safer cell therapies.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
Y. Wang is scientific co-founder of Cell E&G Inc. and Acoustic Cell Therapy Inc. These financial interests do not affect the design, conduct or reporting of this research.
Figures









References
-
- Tsien RY Imagining imaging’s future. Nat. Rev. Mol. Cell Biol 4 (Suppl.), SS16–SS21 (2003). - PubMed
-
- Guilhon E et al. Image-guided control of transgene expression based on local hyperthermia. Mol. Imaging 2, 11–17 (2003). - PubMed
-
- Wang S, Zderic V & Frenkel V Extracorporeal, low-energy focused ultrasound for noninvasive and nondestructive targeted hyperthermia. Future Oncol. 6, 1497–1511 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

