Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial
- PMID: 34385708
- PMCID: PMC8509078
- DOI: 10.1038/s41591-021-01462-y
Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial
Abstract
Adoptive cell therapy using tumor-infiltrating lymphocytes (TILs) has shown activity in melanoma, but has not been previously evaluated in metastatic non-small cell lung cancer. We conducted a single-arm open-label phase 1 trial ( NCT03215810 ) of TILs administered with nivolumab in 20 patients with advanced non-small cell lung cancer following initial progression on nivolumab monotherapy. The primary end point was safety and secondary end points included objective response rate, duration of response and T cell persistence. Autologous TILs were expanded ex vivo from minced tumors cultured with interleukin-2. Patients received cyclophosphamide and fludarabine lymphodepletion, TIL infusion and interleukin-2, followed by maintenance nivolumab. The end point of safety was met according to the prespecified criteria of ≤17% rate of severe toxicity (95% confidence interval, 3-29%). Of 13 evaluable patients, 3 had confirmed responses and 11 had reduction in tumor burden, with a median best change of 35%. Two patients achieved complete responses that were ongoing 1.5 years later. In exploratory analyses, we found T cells recognizing multiple types of cancer mutations were detected after TIL treatment and were enriched in responding patients. Neoantigen-reactive T cell clonotypes increased and persisted in peripheral blood after treatment. Cell therapy with autologous TILs is generally safe and clinically active and may constitute a new treatment strategy in metastatic lung cancer.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interest Statement
The remaining authors have no relevant conflicts to disclose.
Figures
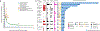
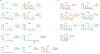

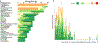
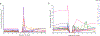





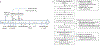


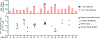
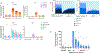
Comment in
-
Tumor-infiltrating lymphocytes make inroads in non-small-cell lung cancer.Nat Med. 2021 Aug;27(8):1339-1341. doi: 10.1038/s41591-021-01445-z. Nat Med. 2021. PMID: 34385706 No abstract available.
-
TILs show early efficacy.Nat Rev Clin Oncol. 2021 Oct;18(10):603. doi: 10.1038/s41571-021-00555-4. Nat Rev Clin Oncol. 2021. PMID: 34453131 No abstract available.
References
-
- Hellmann MD, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. New England Journal of Medicine 381, 2020–2031 (2019). - PubMed
References for Online Methods
-
- Andersen R, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clinical Cancer Research 22, 3734–3745 (2016). - PubMed
-
- Cox J. & Mann M. MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nature biotechnology 26, 1367–1372 (2008). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

