Pathogenic Characterization of Clostridium perfringens Strains Isolated From Patients With Massive Intravascular Hemolysis
- PMID: 34385995
- PMCID: PMC8353389
- DOI: 10.3389/fmicb.2021.713509
Pathogenic Characterization of Clostridium perfringens Strains Isolated From Patients With Massive Intravascular Hemolysis
Abstract
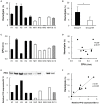
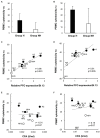
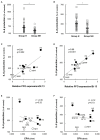
Sepsis caused by Clostridium perfringens infection is rare but often fatal. The most serious complication leading to poor prognosis is massive intravascular hemolysis (MIH). However, the molecular mechanism underlying this fulminant form of hemolysis is unclear. In the present study, we employed 11 clinical strains isolated from patients with C. perfringens septicemia and subdivided these isolates into groups H and NH: septicemia with (n = 5) or without (n = 6) MIH, respectively. To elucidate the major pathogenic factors of MIH, biological features were compared between these groups. The isolates of two groups did not differ in growth rate, virulence-related gene expression, or phospholipase C (CPA) production. Erythrocyte hemolysis was predominantly observed in culture supernatants of the strains in group H, and the human erythrocyte hemolysis rate was significantly correlated with perfringolysin O (PFO) production. Correlations were also found among PFO production, human peripheral blood mononuclear cell (PBMC) cytotoxicity, and production of interleukin-6 (IL-6) and interleukin-8 (IL-8) by human PBMCs. Analysis of proinflammatory cytokines showed that PFO induced tumor necrosis factor-α (TNF-α), IL-5, IL-6, and IL-8 production more strongly than did CPA. PFO exerted potent cytotoxic and proinflammatory cytokine induction effects on human blood cells. PFO may be a major virulence factor of sepsis with MIH, and potent proinflammatory cytokine production induced by PFO may influence the rapid progression of this fatal disease caused by C. perfringens.
Keywords: Clostridium perfringens; cytokine; hemolysis; perfringolysin O; phospholipase C; sepsis.
Copyright © 2021 Suzaki, Ohtani, Komine-Aizawa, Matsumoto, Kamiya and Hayakawa.
Conflict of interest statement
AM and SK are employed by Miyarisan Pharmaceutical Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aisiku I. P., Yamal J. M., Doshi P., Benoit J. S., Gopinath S., Goodman J. C., et al. . (2016). Plasma cytokines IL-6, IL-8, and IL-10 are associated with the development of acute respiratory distress syndrome in patients with severe traumatic brain injury. Crit. Care 20:288. doi: 10.1186/s13054-016-1470-7, PMID: - DOI - PMC - PubMed
-
- Bryant A. E., Bergstrom R., Zimmerman G. A., Salyer J. L., Hill H. R., Tweten R. K., et al. . (1993). Clostridium perfringens invasiveness is enhanced by effects of theta toxin upon PMNL structure and function: the roles of leukocytotoxicity and expression of CD11/CD18 adherence glycoprotein. FEMS Immunol. Med. Microbiol. 7, 321–336. doi: 10.1111/j.1574-695X.1993.tb00414.x, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

