Sequential Deposition and Remodeling of Cell Wall Polymers During Tomato Pollen Development
- PMID: 34386029
- PMCID: PMC8354551
- DOI: 10.3389/fpls.2021.703713
Sequential Deposition and Remodeling of Cell Wall Polymers During Tomato Pollen Development
Abstract
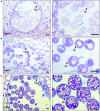
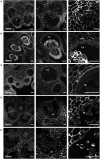
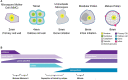
The cell wall of a mature pollen grain is a highly specialized, multilayered structure. The outer, sporopollenin-based exine provides protection and support to the pollen grain, while the inner intine, composed primarily of cellulose, is important for pollen germination. The formation of the mature pollen grain wall takes place within the anther with contributions of cell wall material from both the developing pollen grain as well as the surrounding cells of the tapetum. The process of wall development is complex; multiple cell wall polymers are deposited, some transiently, in a controlled sequence of events. Tomato (Solanum lycopersicum) is an important agricultural crop, which requires successful fertilization for fruit production as do many other members of the Solanaceae family. Despite the importance of pollen development for tomato, little is known about the detailed pollen gain wall developmental process. Here, we describe the structure of the tomato pollen wall and establish a developmental timeline of its formation. Mature tomato pollen is released from the anther in a dehydrated state and is tricolpate, with three long apertures without overlaying exine from which the pollen tube may emerge. Using histology and immunostaining, we determined the order in which key cell wall polymers were deposited with respect to overall pollen and anther development. Pollen development began in young flower buds when the premeiotic microspore mother cells (MMCs) began losing their cellulose primary cell wall. Following meiosis, the still conjoined microspores progressed to the tetrad stage characterized by a temporary, thick callose wall. Breakdown of the callose wall released the individual early microspores. Exine deposition began with the secretion of the sporopollenin foot layer. At the late microspore stage, exine deposition was completed and the tapetum degenerated. The pollen underwent mitosis to produce bicellular pollen; at which point, intine formation began, continuing through to pollen maturation. The entire cell wall development process was also punctuated by dynamic changes in pectin composition, particularly changes in methyl-esterified and de-methyl-esterified homogalacturonan.
Keywords: 3-glucan); callose (β-1; cell wall; cellulose; exine development; intine; pectin; pollen; tomato.
Copyright © 2021 Jaffri and MacAlister.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources

