3D organoids derived from the small intestine: An emerging tool for drug transport research
- PMID: 34386316
- PMCID: PMC8343122
- DOI: 10.1016/j.apsb.2020.12.002
3D organoids derived from the small intestine: An emerging tool for drug transport research
Abstract
Small intestine in vitro models play a crucial role in drug transport research. Although conventional 2D cell culture models, such as Caco-2 monolayer, possess many advantages, they should be interpreted with caution because they have relatively poor physiologically reproducible phenotypes and functions. With the development of 3D culture technology, pluripotent stem cells (PSCs) and adult somatic stem cells (ASCs) show remarkable self-organization characteristics, which leads to the development of intestinal organoids. Based on previous studies, this paper reviews the application of intestinal 3D organoids in drug transport mediated by P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and multidrug resistance protein 2 (MRP2). The advantages and limitations of this model are also discussed. Although there are still many challenges, intestinal 3D organoid model has the potential to be an excellent tool for drug transport research.
Keywords: 3D organoid; ASCs, adult somatic stem cells; BCRP, breast cancer resistance protein; BMP, bone morphogenetic protein; CDF, 5(6)-carboxy-2′,7′-dichlorofluorescein; Caco-2 cell monolayer; DDI, drug–drug interactions; Drug transporter; EGF, epidermal growth factor; ER, efflux ratio; ESCs, embryonic stem cells; FGF, fibroblast growth factor; Lgr5+, leucine-rich-repeat-containing G-protein-coupled receptor 5 positive; MCT, monocarboxylate transporter protein; MRP2, multidrug resistance protein 2; NBD, nucleotide-binding domain; OATP, organic anion transporting polypeptide; OCT, organic cation transporter; OCTN, carnitine/organic cation transporter; P-glycoprotein; P-gp, P-glycoprotein; PEPT, peptide transporter protein; PMAT, plasma membrane monoamine transporter; PSCs, pluripotent stem cells; Papp, apparent permeability coefficient; Rh123, rhodamine 123; SLC, solute carrier; Small intestine; TEER, transepithelial electrical resistance; TMDs, transmembrane domains; cMOAT, canalicular multispecific organic anion transporter; iPSCs, induced pluripotent stem cells.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures
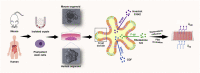
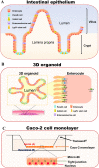
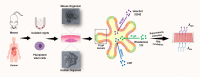
Similar articles
-
Understanding peroral absorption: regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles.Acta Pharm Sin B. 2017 May;7(3):260-280. doi: 10.1016/j.apsb.2016.09.005. Epub 2016 Nov 2. Acta Pharm Sin B. 2017. PMID: 28540164 Free PMC article. Review.
-
Characterization of in vitro Mrp2 transporter model based on intestinal organoids.Regul Toxicol Pharmacol. 2019 Nov;108:104449. doi: 10.1016/j.yrtph.2019.104449. Epub 2019 Aug 23. Regul Toxicol Pharmacol. 2019. PMID: 31449916
-
Role of P-glycoprotein in the efflux of raltegravir from human intestinal cells and CD4+ T-cells as an interaction target for anti-HIV agents.Biochem Biophys Res Commun. 2013 Sep 20;439(2):221-7. doi: 10.1016/j.bbrc.2013.08.054. Epub 2013 Aug 24. Biochem Biophys Res Commun. 2013. PMID: 23981805
-
Polarized efflux of mono- and diacid metabolites of ME3229, an ester-type prodrug of a glycoprotein IIb/IIIa receptor antagonist, in rat small intestine.J Pharmacol Exp Ther. 2000 Nov;295(2):717-23. J Pharmacol Exp Ther. 2000. PMID: 11046110
-
Renal drug transporters and their significance in drug-drug interactions.Acta Pharm Sin B. 2016 Sep;6(5):363-373. doi: 10.1016/j.apsb.2016.07.013. Epub 2016 Aug 9. Acta Pharm Sin B. 2016. PMID: 27709005 Free PMC article. Review.
Cited by
-
Liver organoids: an in vitro 3D model for liver cancer study.Cell Biosci. 2022 Sep 9;12(1):152. doi: 10.1186/s13578-022-00890-8. Cell Biosci. 2022. PMID: 36085085 Free PMC article. Review.
-
A 3D co-culture intestinal organoid system for exploring glucose metabolism.Curr Res Food Sci. 2022 Nov 28;6:100402. doi: 10.1016/j.crfs.2022.11.021. eCollection 2023. Curr Res Food Sci. 2022. PMID: 36479229 Free PMC article. Review.
-
Establishment and evaluation of on-chip intestinal barrier biosystems based on microfluidic techniques.Mater Today Bio. 2024 May 5;26:101079. doi: 10.1016/j.mtbio.2024.101079. eCollection 2024 Jun. Mater Today Bio. 2024. PMID: 38774450 Free PMC article. Review.
-
Potential consequences of phototoxicity on cell function during live imaging of intestinal organoids.PLoS One. 2024 Nov 15;19(11):e0313213. doi: 10.1371/journal.pone.0313213. eCollection 2024. PLoS One. 2024. PMID: 39546479 Free PMC article.
-
Mouse Small Intestinal Organoid Cultures.Methods Mol Biol. 2025;2951:77-90. doi: 10.1007/7651_2024_576. Methods Mol Biol. 2025. PMID: 39570547
References
-
- Li A.P. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov Today. 2001;6:357–366. - PubMed
-
- Sarmento B., Andrade F., da Silva S.B., Rodrigues F., das Neves J., Ferreira D. Cell-based in vitro models for predicting drug permeability. Expet Opin Drug Metabol Toxicol. 2012;8:607–621. - PubMed
-
- Antunes F., Andrade F., Ferreira D., Nielsen H.M., Sarmento B. Models to predict intestinal absorption of therapeutic peptides and proteins. Curr Drug Metabol. 2013;14:4–20. - PubMed
-
- Dutta D., Heo I., Clevers H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med. 2017;23:393–410. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

