Targeted inhibition of GRK2 kinase domain by CP-25 to reverse fibroblast-like synoviocytes dysfunction and improve collagen-induced arthritis in rats
- PMID: 34386323
- PMCID: PMC8343125
- DOI: 10.1016/j.apsb.2021.01.015
Targeted inhibition of GRK2 kinase domain by CP-25 to reverse fibroblast-like synoviocytes dysfunction and improve collagen-induced arthritis in rats
Abstract
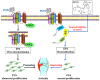
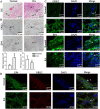
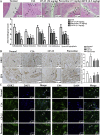
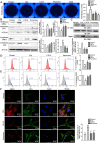
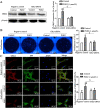
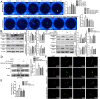
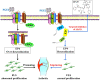
Rheumatoid arthritis (RA) is an autoimmune disease and is mainly characterized by abnormal proliferation of fibroblast-like synoviocytes (FLS). The up-regulated cellular membrane expression of G protein coupled receptor kinase 2 (GRK2) of FLS plays a critical role in RA progression, the increase of GRK2 translocation activity promotes dysfunctional prostaglandin E4 receptor (EP4) signaling and FLS abnormal proliferation. Recently, although our group found that paeoniflorin-6'-O-benzene sulfonate (CP-25), a novel compound, could reverse FLS dysfunction via GRK2, little is known as to how GRK2 translocation activity is suppressed. Our findings revealed that GRK2 expression up-regulated and EP4 expression down-regulated in synovial tissues of RA patients and collagen-induced arthritis (CIA) rats, and prostaglandin E2 (PGE2) level increased in arthritis. CP-25 could down-regulate GRK2 expression, up-regulate EP4 expression, and improve synovitis of CIA rats. CP-25 and GRK2 inhibitors (paroxetine or GSK180736A) inhibited the abnormal proliferation of FLS in RA patients and CIA rats by down-regulating GRK2 translocation to EP4 receptor. The results of microscale thermophoresis (MST), cellular thermal shift assay, and inhibition of kinase activity assay indicated that CP-25 could directly target GRK2, increase the protein stability of GRK2 in cells, and inhibit GRK2 kinase activity. The docking of CP-25 and GRK2 suggested that the kinase domain of GRK2 might be an important active pocket for CP-25. G201, K220, K230, A321, and D335 in kinase domain of GRK2 might form hydrogen bonds with CP-25. Site-directed mutagenesis and co-immunoprecipitation assay further revealed that CP-25 down-regulated the interaction of GRK2 and EP4 via controlling the key amino acid residue of Ala321 of GRK2. Our data demonstrate that FLS proliferation is regulated by GRK2 translocation to EP4. Targeted inhibition of GRK2 kinase domain by CP-25 improves FLS function and represents an innovative drug for the treatment of RA by targeting GRK2.
Keywords: CP-25; Fibroblast-like synoviocyte; G protein coupled receptor kinase 2; MH7A; Prostaglandin E4 receptor; Rheumatoid arthritis.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


References
-
- Cross M., Smith E., Hoy D., Carmona L., Wolfe F., Vos T. The global burden of rheumatoid arthritis: Estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1316–1322. - PubMed
-
- Benson R.A., McInnes I.B., Brewer J.M., Garside P. Cellular imaging in rheumatic diseases. Nat Rev Rheumatol. 2015;11:357–367. - PubMed
-
- Schumacher H.R. Are we being open enough to all approaches to therapy of rheumatoid arthritis?. J Clin Rheumatol. 2013;19:167–171. - PubMed
-
- Lee D.M., Kiener H.P., Agarwal S.K., Noss E.H., Watts G.F.M., Chisaka O. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. - PubMed
-
- Chen Q., Wei W. Effects and mechanisms of glucosides of chaenomeles speciosa on collagen-induced arthritis in rats. Int Immunopharm. 2003;3:593–608. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

