Neutrophils require SKAP2 for reactive oxygen species production following C-type lectin and Candida stimulation
- PMID: 34386732
- PMCID: PMC8346660
- DOI: 10.1016/j.isci.2021.102871
Neutrophils require SKAP2 for reactive oxygen species production following C-type lectin and Candida stimulation
Abstract
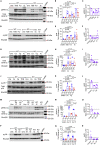
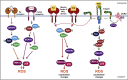
Signaling cascades converting the recognition of pathogens to efficient inflammatory responses by neutrophils are critical for host survival. SKAP2, an adaptor protein, is required for reactive oxygen species (ROS) generation following neutrophil stimulation by integrins, formyl peptide receptors, and for host defense against the Gram-negative bacterial pathogens, Klebsiella pneumoniae and Yersinia pseudotuberculosis. Using neutrophils from murine HoxB8-immortalized progenitors, we show that SKAP2 in neutrophils is crucial for maximal ROS response to purified C-type lectin receptor agonists and to the fungal pathogens, Candida glabrata and Candida albicans, and for robust killing of C. glabrata. Inside-out signaling to integrin and Syk phosphorylation occurred independently of SKAP2 after Candida infection. However, Pyk2, ERK1/2, and p38 phosphorylation were significantly reduced after infection with C. glabrata and K. pneumoniae in Skap2-/- neutrophils. These data demonstrate the importance of SKAP2 in ROS generation and host defense beyond antibacterial immunity to include CLRs and Candida species.
Keywords: Biological sciences; Immunology; Microbiology; cell biology.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Adrover J.M., Nicolas-Avila J.A., Hidalgo A. Aging: a temporal dimension for neutrophils. Trends Immunol. 2016;37:334–345. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

