Dendritic Kv4.2 potassium channels selectively mediate spatial pattern separation in the dentate gyrus
- PMID: 34386734
- PMCID: PMC8346659
- DOI: 10.1016/j.isci.2021.102876
Dendritic Kv4.2 potassium channels selectively mediate spatial pattern separation in the dentate gyrus
Abstract
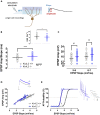
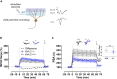
The capacity to distinguish comparable experiences is fundamental for the recall of similar memories and has been proposed to require pattern separation in the dentate gyrus (DG). However, the cellular mechanisms by which mature granule cells (GCs) of the DG accomplish this function are poorly characterized. Here, we show that Kv4.2 channels selectively modulate the excitability of medial dendrites of dentate GCs. These dendrites are targeted by the medial entorhinal cortex, the main source of spatial inputs to the DG. Accordingly, we found that the spatial pattern separation capability of animals lacking the Kv4.2 channel is significantly impaired. This points to the role of intrinsic excitability in supporting the mnemonic function of the dentate and to the Kv4.2 channel as a candidate substrate promoting spatial pattern separation.
Keywords: Biological sciences; Cellular neuroscience; Neuroscience.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Alme C.B., Buzzetti R.A., Marrone D.F., Leutgeb J.K., Chawla M.K., Schaner M.J., Bohanick J.D., Khoboko T., Leutgeb S., Moser E.I. Hippocampal granule cells opt for early retirement. Hippocampus. 2010;20:1109–1123. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

