Association Between Patient Survival and Clinician Variability in Treatment Rates for Aortic Valve Stenosis
- PMID: 34387116
- PMCID: PMC8475044
- DOI: 10.1161/JAHA.120.020490
Association Between Patient Survival and Clinician Variability in Treatment Rates for Aortic Valve Stenosis
Abstract
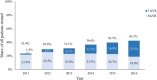
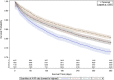
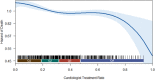
Background Patients with symptomatic severe aortic stenosis (ssAS) have a high mortality risk and compromised quality of life. Surgical/transcatheter aortic valve replacement (AVR) is a Class I recommendation, but it is unclear if this recommendation is uniformly applied. We determined the impact of managing cardiologists on the likelihood of ssAS treatment. Methods and Results Using natural language processing of Optum electronic health records, we identified 26 438 patients with newly diagnosed ssAS (2011-2016). Multilevel, multivariable Fine-Gray competing risk models clustered by cardiologists were used to determine the impact of cardiologists on the likelihood of 1-year AVR treatment. Within 1 year of diagnosis, 35.6% of patients with ssAS received an AVR; however, rates varied widely among managing cardiologists (0%, lowest quartile; 100%, highest quartile [median, 29.6%; 25th-75th percentiles, 13.3%-47.0%]). The odds of receiving AVR varied >2-fold depending on the cardiologist (median odds ratio for AVR, 2.25; 95% CI, 2.14-2.36). Compared with patients with ssAS of cardiologists with the highest treatment rates, those treated by cardiologists with the lowest AVR rates experienced significantly higher 1-year mortality (lowest quartile, adjusted hazard ratio, 1.22, 95% CI, 1.13-1.33). Conclusions Overall AVR rates for ssAS were low, highlighting a potential challenge for ssAS management in the United States. Cardiologist AVR use varied substantially; patients treated by cardiologists with lower AVR rates had higher mortality rates than those treated by cardiologists with higher AVR rates.
Keywords: aortic valve replacement; physician variability; symptomatic severe aortic stenosis.
Conflict of interest statement
Dr Brennan reports consulting and speaking funds from Edwards Lifesciences and AtriCure. Dr Boero reports consulting for Edwards Lifesciences. Dr Thourani reports research and advising for Edwards Lifesciences. Dr Vemulapalli reports grants/contracts from the American College of Cardiology, Society of Thoracic Surgeons, Abbott Vascular, Boston Scientific, National Institutes of Health (R01 and Small Business Innovation Research grants), Food and Drug Administration National Evaluation System for health Technology Coordinating Center (FDA NESTcc), and Cytokinetics and advisory board/consulting/honoraria with Boston Scientific, American College of Physicians, Janssen, Edwards Lifesciences, and HeartFlow. Dr Wang reports research grants to the Duke Clinical Research Institute from Abbott, AstraZeneca, Bristol Myers Squibb, Boston Scientific, Cryolife, Chiesi, Merck, Portola, and Regeneron and consulting honoraria from AstraZeneca, Bristol Myers Squibb, Cryolife, and Novartis. Mr Liska, Mr Gander, and Mr Jager report consulting for Edwards Lifesciences. Dr Peterson reports being a coinvestigator on the American College of Cardiology Society of Thoracic Surgeons Transcatheter Valve Therapy TAVR Registry. The remaining authors have no disclosures to report.
Figures

References
-
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. DOI: 10.1161/CIR.0000000000000503. - DOI - PubMed
-
- Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, et al.; PARTNER 1 Trial Investigators . 5‐year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484. DOI: 10.1016/S0140-6736(15)60308-7. - DOI - PubMed
-
- Baron SJ, Arnold SV, Wang K, Magnuson EA, Chinnakondepali K, Makkar R, Herrmann HC, Kodali S, Thourani VH, Kapadia S, et al.; PARTNER 2 Investigators . Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2:837–845. DOI: 10.1001/jamacardio.2017.2039. - DOI - PMC - PubMed
-
- ClinicalTrials.gov . PARTNER 3 trial: the safety and effectiveness of the SAPIEN 3 transcatheter heart valve in low risk patients with aortic stenosis (P3). ClinicalTrials.gov web site. Updated April 8, 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT02675114. Accessed November 1, 2019.
-
- ClinicalTrials.gov Medtronic evolut transcatheter aortic valve replacement in low risk patients. ClinicalTrials.gov web site. Available at: https://clinicaltrials.gov/ct2/show/NCT02701283. Updated October 30, 2019. Accessed November 1, 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

