Non-nucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy is associated with lower cell-associated HIV RNA and DNA levels compared to protease inhibitor-based therapy
- PMID: 34387543
- PMCID: PMC8460250
- DOI: 10.7554/eLife.68174
Non-nucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy is associated with lower cell-associated HIV RNA and DNA levels compared to protease inhibitor-based therapy
Abstract
Background: It remains unclear whether combination antiretroviral therapy (ART) regimens differ in their ability to fully suppress human immunodeficiency virus (HIV) replication. Here, we report the results of two cross-sectional studies that compared levels of cell-associated (CA) HIV markers between individuals receiving suppressive ART containing either a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor (PI).
Methods: CA HIV unspliced RNA and total HIV DNA were quantified in two cohorts (n = 100, n = 124) of individuals treated with triple ART regimens consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) plus either an NNRTI or a PI. To compare CA HIV RNA and DNA levels between the regimens, we built multivariable models adjusting for age, gender, current and nadir CD4+ count, plasma viral load zenith, duration of virological suppression, NRTI backbone composition, low-level plasma HIV RNA detectability, and electronically measured adherence to ART.
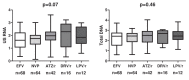
Results: In both cohorts, levels of CA HIV RNA and DNA strongly correlated (rho = 0.70 and rho = 0.54) and both markers were lower in NNRTI-treated than in PI-treated individuals. In the multivariable analysis, CA RNA in both cohorts remained significantly reduced in NNRTI-treated individuals (padj = 0.02 in both cohorts), with a similar but weaker association between the ART regimen and total HIV DNA (padj = 0.048 and padj = 0.10). No differences in CA HIV RNA or DNA levels were observed between individual NNRTIs or individual PIs, but CA HIV RNA was lower in individuals treated with either nevirapine or efavirenz, compared to PI-treated individuals.
Conclusions: All current classes of antiretroviral drugs only prevent infection of new cells but do not inhibit HIV RNA transcription in long-lived reservoir cells. Therefore, these differences in CA HIV RNA and DNA levels by treatment regimen suggest that NNRTIs are more potent in suppressing HIV residual replication than PIs, which may result in a smaller viral reservoir size.
Funding: This work was supported by ZonMw (09120011910035) and FP7 Health (305522).
Keywords: HIV; antiviral drugs; infectious disease; medicine; microbiology; virus; virus infection.
© 2021, Pasternak et al.
Conflict of interest statement
AP, JV, NK, FW, Md, DD, MB, CS, AW, JP, PR, BB No competing interests declared
Figures

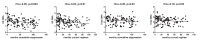






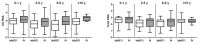
References
-
- Abdel-Mohsen M, Richman D, Siliciano RF, Nussenzweig MC, Howell BJ, Martinez-Picado J, Chomont N, Bar KJ, Yu XG, Lichterfeld M, Alcami J, Hazuda D, Bushman F, Siliciano JD, Betts MR, Spivak AM, Planelles V, Hahn BH, Smith DM, Ho YC, Buzon MJ, Gaebler C, Paiardini M, Li Q, Estes JD, Hope TJ, Kostman J, Mounzer K, Caskey M, Fox L, Frank I, Riley JL, Tebas P, Montaner LJ, BEAT-HIV Delaney Collaboratory to Cure HIV-1 infection Recommendations for measuring HIV reservoir size in cure-directed clinical trials. Nature Medicine. 2020;26:1339–1350. doi: 10.1038/s41591-020-1022-1. - DOI - PMC - PubMed
-
- Abrahams MR, Joseph SB, Garrett N, Tyers L, Moeser M, Archin N, Council OD, Matten D, Zhou S, Doolabh D, Anthony C, Goonetilleke N, Karim SA, Margolis DM, Pond SK, Williamson C, Swanstrom R. The replication-competent HIV-1 latent reservoir is primarily established near the time of therapy initiation. Science Translational Medicine. 2019;11:eaaw5589. doi: 10.1126/scitranslmed.aaw5589. - DOI - PMC - PubMed
-
- Bachmann N, von Siebenthal C, Vongrad V, Turk T, Neumann K, Beerenwinkel N, Bogojeska J, Fellay J, Roth V, Kok YL, Thorball CW, Borghesi A, Parbhoo S, Wieser M, Böni J, Perreau M, Klimkait T, Yerly S, Battegay M, Rauch A, Hoffmann M, Bernasconi E, Cavassini M, Kouyos RD, Günthard HF, Metzner KJ, Swiss HIV Cohort Study Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nature Communications. 2019;10:3193. doi: 10.1038/s41467-019-10884-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

