Safety of Lubiprostone in Pediatric Patients With Functional Constipation: A Nonrandomized, Open-Label Trial
- PMID: 34387619
- PMCID: PMC8528133
- DOI: 10.1097/MPG.0000000000003280
Safety of Lubiprostone in Pediatric Patients With Functional Constipation: A Nonrandomized, Open-Label Trial
Abstract
Objectives: Pediatric functional constipation (PFC) affects up to 30% of children. Current treatments often do not sustain symptomatic relief. Lubiprostone is a locally acting chloride channel activator that promotes fluid secretion into the small bowel without affecting serum electrolyte concentrations. We assessed the safety/tolerability of oral lubiprostone as treatment for PFC in a 24-week study.
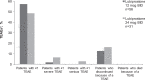
Methods: This phase 3 open-label safety trial conducted from April-November 2016 at 13 US sites included patients (ages 6-17 years) diagnosed with PFC (Rome III criteria). Patients <50 and ≥50 kg received lubiprostone 12 or 24 mcg twice daily, respectively, for 24 weeks. Safety endpoints included incidence of treatment-emergent adverse events (TEAEs) and changes from baseline in clinical laboratory parameters and vital signs.
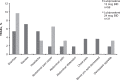
Results: Overall, 87 patients receiving lubiprostone, 64.3% (36/56) in the 12-mcg group and 54.8% (17/31) in the 24-mcg group, completed the study. Of 12 TEAEs leading to discontinuation, only upper abdominal pain occurred in >1 patient. TEAEs were mostly mild in intensity, with gastrointestinal disorders (diarrhea, vomiting) most frequently reported. No safety concerns were found in vital signs, abbreviated physical examinations, and laboratory tests. Subgroup analyses assessed an impact of age, sex, and race categories on TEAEs and treatment-related adverse events. Mean investigators' assessments of treatment effectiveness (scale of 0-4) for lubiprostone 12- and 24-mcg groups, respectively, were 2.8 and 2.9 at week 12, and 2.7 and 2.2 at week 24.
Conclusions: Lubiprostone was well tolerated in the pediatric population. The incidence of TEAEs was comparable to that observed in previous clinical trials and in adults.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
Conflict of interest statement
Conflicts of Interest: S.Z.H. was a consultant for Sucampo Pharmaceuticals (now Mallinckrodt Pharmaceuticals). S.M. is a former employee of Sucampo Pharmaceuticals (now Mallinckrodt Pharmaceuticals); had stock options in Sucampo Pharmaceuticals before acquisition by Mallinckrodt Pharmaceuticals. The other authors report no conflict of interest.
Figures
References
-
- van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol 2006; 101:2401–2409. - PubMed
-
- Tabbers MM, DiLorenzo C, Berger MY, et al. . Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014; 58:258–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

