Change in Saliva RT-PCR Sensitivity Over the Course of SARS-CoV-2 Infection
- PMID: 34387653
- PMCID: PMC8456387
- DOI: 10.1001/jama.2021.13967
Change in Saliva RT-PCR Sensitivity Over the Course of SARS-CoV-2 Infection
Abstract
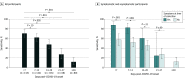
This study investigates the timeframe that optimizes saliva sensitivity for SARS-CoV-2 detection using reverse transcriptase–polymerase chain reaction (RT-PCR) testing.
Conflict of interest statement
Figures
Comment in
-
Saliva RT-PCR Sensitivity Over the Course of SARS-CoV-2 Infection.JAMA. 2022 Jan 11;327(2):182-183. doi: 10.1001/jama.2021.21703. JAMA. 2022. PMID: 35015044 No abstract available.
Similar articles
-
Assessment of Multiplex Digital Droplet RT-PCR as a Diagnostic Tool for SARS-CoV-2 Detection in Nasopharyngeal Swabs and Saliva Samples.Clin Chem. 2021 Apr 29;67(5):736-741. doi: 10.1093/clinchem/hvaa323. Clin Chem. 2021. PMID: 33331864 Free PMC article.
-
A Multiplex One-Step RT-qPCR Protocol to Detect SARS-CoV-2 in NP/OP Swabs and Saliva.Curr Protoc. 2021 May;1(5):e145. doi: 10.1002/cpz1.145. Curr Protoc. 2021. PMID: 34004070 Free PMC article.
-
Pooling of nasopharyngeal swab specimens for SARS-CoV-2 detection by RT-PCR.J Med Virol. 2020 Nov;92(11):2306-2307. doi: 10.1002/jmv.25971. Epub 2020 Jun 2. J Med Virol. 2020. PMID: 32369202 Free PMC article. No abstract available.
-
Concentrating Pooled COVID-19 Patient Lysates to Improve Reverse Transcription Quantitative PCR Sensitivity and Efficiency.Clin Chem. 2021 Apr 29;67(5):797-798. doi: 10.1093/clinchem/hvab035. Clin Chem. 2021. PMID: 33822907 Free PMC article. No abstract available.
-
Alternative clinical specimens for the detection of SARS-CoV-2: A rapid review.Rev Med Virol. 2021 Jul;31(4):e2185. doi: 10.1002/rmv.2185. Epub 2020 Oct 22. Rev Med Virol. 2021. PMID: 33091200 Review.
Cited by
-
Omicron Wave SARS-CoV-2 Diagnosis: Evaluation of Saliva, Anterior Nasal, and Nasopharyngeal Swab Samples.Microbiol Spectr. 2022 Dec 21;10(6):e0252122. doi: 10.1128/spectrum.02521-22. Epub 2022 Nov 1. Microbiol Spectr. 2022. PMID: 36318040 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Update of Guidelines for Laboratory Diagnosis of COVID-19 in Korea.Ann Lab Med. 2022 Jul 1;42(4):391-397. doi: 10.3343/alm.2022.42.4.391. Ann Lab Med. 2022. PMID: 35177559 Free PMC article.
-
Identification of SARS-CoV-2 variants using viral sequencing for the Centers for Disease Control and Prevention genomic surveillance program.BMC Infect Dis. 2022 Apr 25;22(1):404. doi: 10.1186/s12879-022-07374-7. BMC Infect Dis. 2022. PMID: 35468749 Free PMC article.
-
Self-Collected Samples to Detect SARS-CoV-2: Direct Comparison of Saliva, Tongue Swab, Nasal Swab, Chewed Cotton Pads and Gargle Lavage.J Clin Med. 2021 Dec 8;10(24):5751. doi: 10.3390/jcm10245751. J Clin Med. 2021. PMID: 34945047 Free PMC article.
References
-
- Centers for Disease Control and Prevention . Interim guidelines for collecting and handling of clinical specimens for COVID-19 testing. Accessed February 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim...
-
- Jaafar R, Aherfi S, Wurtz N, et al. . Correlation between 3790 quantitative polymerase chain reaction-positives samples and positive cell cultures including 1941 severe acute respiratory syndrome coronavirus 2 isolates. Clin Infect Dis. 2021;72(11):e921. doi:10.1093/cid/ciaa1491 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

