Digital Reconstruction of the Neuro-Glia-Vascular Architecture
- PMID: 34387659
- PMCID: PMC8568010
- DOI: 10.1093/cercor/bhab254
Digital Reconstruction of the Neuro-Glia-Vascular Architecture
Abstract
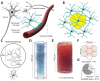
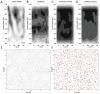
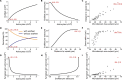
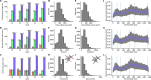
Astrocytes connect the vasculature to neurons mediating the supply of nutrients and biochemicals. They are involved in a growing number of physiological and pathophysiological processes that result from biophysical, physiological, and molecular interactions in this neuro-glia-vascular ensemble (NGV). The lack of a detailed cytoarchitecture severely restricts the understanding of how they support brain function. To address this problem, we used data from multiple sources to create a data-driven digital reconstruction of the NGV at micrometer anatomical resolution. We reconstructed 0.2 mm3 of the rat somatosensory cortex with 16 000 morphologically detailed neurons, 2500 protoplasmic astrocytes, and its microvasculature. The consistency of the reconstruction with a wide array of experimental measurements allows novel predictions of the NGV organization, allowing the anatomical reconstruction of overlapping astrocytic microdomains and the quantification of endfeet connecting each astrocyte to the vasculature, as well as the extent to which they cover the latter. Structural analysis showed that astrocytes optimize their positions to provide uniform vascular coverage for trophic support and signaling. However, this optimal organization rapidly declines as their density increases. The NGV digital reconstruction is a resource that will enable a better understanding of the anatomical principles and geometric constraints, which govern how astrocytes support brain function.
Keywords: 3D models; astrocyte; morphology; neuro-glia-vasculature; neuroanatomy; simulation.
© The Author(s) 2021. Published by Oxford University Press.
Figures




References
-
- Abbott NJ, Rönnbäck L, Hansson E. 2006. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 7(1):41–53. - PubMed
-
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. 2010. Structure and function of the blood–brain barrier. Neurobiol Dis. 37:13–25. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22(5):208–215. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

