Comparison of Antiviral Agents for Seasonal Influenza Outcomes in Healthy Adults and Children: A Systematic Review and Network Meta-analysis
- PMID: 34387680
- PMCID: PMC8363918
- DOI: 10.1001/jamanetworkopen.2021.19151
Comparison of Antiviral Agents for Seasonal Influenza Outcomes in Healthy Adults and Children: A Systematic Review and Network Meta-analysis
Erratum in
-
Error in Figure Caption.JAMA Netw Open. 2021 Oct 1;4(10):e2133433. doi: 10.1001/jamanetworkopen.2021.33433. JAMA Netw Open. 2021. PMID: 34613408 Free PMC article. No abstract available.
Abstract
Importance: Antiviral treatment of influenza is recommended for patients with influenza-like illness during periods of community cocirculation of influenza viruses and SARS-CoV-2; however, questions remain about which treatment is associated with the best outcomes and fewest adverse events.
Objective: To compare the efficacy and safety of neuraminidase inhibitors and the endonuclease inhibitor for the treatment of seasonal influenza among healthy adults and children.
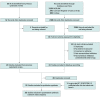
Data sources: Medline, Embase, and the Cochrane Register of Clinical Trials were searched from inception to January 2020 (the last search was updated in October 2020).
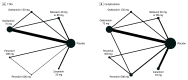
Study selection: Included studies were randomized clinical trials conducted among patients of all ages with influenza treated with neuraminidase inhibitors (ie, oseltamivir, peramivir, zanamivir, or laninamivir) or an endonuclease inhibitor (ie, baloxavir) compared with other active agents or placebo.
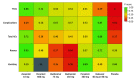
Data extraction and synthesis: Two investigators identified studies and independently abstracted data. Frequentist network meta-analyses were performed; relative ranking of agents was conducted using P-score probabilities. Quality of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations criteria. Data were analyzed in October 2020.
Main outcomes and measures: The time to alleviation of influenza symptoms (TTAS), complications of influenza, and adverse events (total adverse events, nausea, and vomiting).
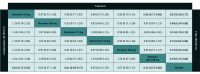
Results: A total of 26 trials were identified that investigated antiviral drugs at high or low doses; these trials included 11 897 participants, among whom 6294 (52.9%) were men and the mean (SD) age was 32.5 (16.9) years. Of all treatments comparing with placebo in efficacy outcomes, high-quality evidence indicated that zanamivir was associated with the shortest TTAS (hazard ratio, 0.67; 95% CI, 0.58-0.77), while baloxavir was associated with the lowest risk of influenza-related complications (risk ratio [RR], 0.51; 95% CI, 0.32-0.80) based on moderate-quality evidence. In safety outcomes, baloxavir was associated with the lowest risk of total adverse events (RR, 0.84; 95% CI, 0.74-0.96) compared with placebo based on moderate-quality evidence. There was no strong evidence of associations with risk of nausea or vomiting among all comparisons, except for 75 mg oseltamivir, which was associated with greater occurrence of nausea (RR, 1.82; 95% CI, 1.38-2.41) and vomiting (RR, 1.88; 95% CI, 1.47-2.41).
Conclusions and relevance: In this systematic review and network meta-analysis, all 4 antiviral agents assessed were associated with shortening TTAS; zanamivir was associated with the shortest TTAS, and baloxavir was associated with reduced rate of influenza-related complications.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention . Influenza antiviral medications: summary for clinicians. Accessed December 20, 2020. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm
-
- Uyeki TM, Bernstein HH, Bradley JS, et al. . Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1-e47. doi:10.1093/cid/ciy866 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

