Efficacy and safety of N-acetyl-L-leucine in Niemann-Pick disease type C
- PMID: 34387740
- PMCID: PMC8361244
- DOI: 10.1007/s00415-021-10717-0
Efficacy and safety of N-acetyl-L-leucine in Niemann-Pick disease type C
Abstract
Objective: To investigate the safety and efficacy of N-acetyl-L-leucine (NALL) on symptoms, functioning, and quality of life in pediatric (≥ 6 years) and adult Niemann-Pick disease type C (NPC) patients.
Methods: In this multi-national, open-label, rater-blinded Phase II study, patients were assessed during a baseline period, a 6-week treatment period (orally administered NALL 4 g/day in patients ≥ 13 years, weight-tiered doses for patients 6-12 years), and a 6-week post-treatment washout period. The primary Clinical Impression of Change in Severity (CI-CS) endpoint (based on a 7-point Likert scale) was assessed by blinded, centralized raters who compared randomized video pairs of each patient performing a pre-defined primary anchor test (8-Meter Walk Test or 9-Hole Peg Test) during each study periods. Secondary outcomes included cerebellar functional rating scales, clinical global impression, and quality of life assessments.
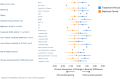
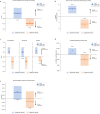
Results: 33 subjects aged 7-64 years with a confirmed diagnosis of NPC were enrolled. 32 patients were included in the primary modified intention-to-treat analysis. NALL met the CI-CS primary endpoint (mean difference 0.86, SD = 2.52, 90% CI 0.25, 1.75, p = 0.029), as well as secondary endpoints. No treatment-related serious adverse events occurred.
Conclusions: NALL demonstrated a statistically significant and clinical meaningfully improvement in symptoms, functioning, and quality of life in 6 weeks, the clinical effect of which was lost after the 6-week washout period. NALL was safe and well-tolerated, informing a favorable benefit-risk profile for the treatment of NPC. CLINICALTRIALS.
Gov identifier: NCT03759639.
Keywords: Acetyl-leucine; Ataxia; Lysosomal storage disorder; Niemann–Pick disease type C; Symptomatic therapy.
© 2021. The Author(s).
Conflict of interest statement
TBE received honoraria for lecturing from Actelion and Sanofi Genzyme and fees for the blinded rater services from IntraBio. JC and JR received fees for the blinded rater services from IntraBio. AH has received previous support from AbbVie and NCATS, and serves on the Editorial Board of Parkinsonism and Related Disorders. UR has received research grants from Amicus and Takeda, advisory board and lecture fee from Amicus, Sanofi Genzyme, and Takeda; all other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

