Vaccination versus infection with SARS-CoV-2: Establishment of a high avidity IgG response versus incomplete avidity maturation
- PMID: 34387884
- PMCID: PMC8427118
- DOI: 10.1002/jmv.27270
Vaccination versus infection with SARS-CoV-2: Establishment of a high avidity IgG response versus incomplete avidity maturation
Abstract
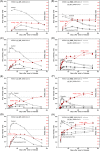
Avidity is defined as the binding strength of immunoglobulin G (IgG) toward its target epitope. Avidity is directly related to affinity, as both processes are determined by the best fit of IgG to epitopes. We confirm and extend data on incomplete avidity maturation of IgG toward severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleoprotein (NP), spike protein-1 (S1), and its receptor-binding domain (RBD) in coronavirus disease 2019 (COVID-19) patients. In SARS-CoV-2-infected individuals, an initial rise in avidity maturation was ending abruptly, leading to IgG of persistently low or intermediate avidity. Incomplete avidity maturation might facilitate secondary SARS-CoV-2 infections and thus prevent the establishment of herd immunity. Incomplete avidity maturation after infection with SARS-CoV-2 (with only 11.8% of cases showing finally IgG of high avidity, that is, an avidity index > 0.6) was contrasted by regular and rapid establishment of high avidity in SARS-CoV-2 naïve individuals after two vaccination steps with the BioNTech messenger RNA (mRNA) Vaccine (78% of cases with high avidity). One vaccination step was not sufficient for induction of complete avidity maturation in vaccinated SARS-CoV-2 naïve individuals, as it induced high avidity only in 2.9% of cases within 3 weeks. However, one vaccination step was sufficient to induce high avidity in individuals with previous SARS-CoV-2 infection.
Keywords: SARS-CoV-2; avidity; protective immunity; receptor-binding domain; recomLine SARS-CoV-2 IgG; vaccination.
© 2021 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
E. Soutschek and M. Motz are owners of Mikrogen GmbH. E. Soutschek is the present CEO of Mikrogen GmbH. F. Struck, E. Stachik, K. Wochinz‐Richter, S. Schulz, and P. Schreiner are employees of Mikrogen GmbH. The determination of avidity of antibodies, using immunoblots or other techniques that allow the parallel measurement of humoral immune reactions toward several antigens in one assay, has been patented by Mikrogen GmbH (WO 00/54055; PCT/EP00/01883). In addition, a new patent application for a method to determine the antibodies toward SARS‐CoV‐2 has been filed by Mikrogen GmbH and is pending (EP 2019/2550). Mikrogen GmbH develops and produces test systems for serological analysis of infectious diseases. G. Bauer is a member of the Medical Faculty of the University of Freiburg. He is the inventor of WO 00/54055; PCT/EP00/01883 and one of the coinventors of EP 2019/2550.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

