YAP-TEAD mediates PPAR α-induced hepatomegaly and liver regeneration in mice
- PMID: 34387904
- PMCID: PMC10259205
- DOI: 10.1002/hep.32105
YAP-TEAD mediates PPAR α-induced hepatomegaly and liver regeneration in mice
Abstract
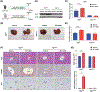
Background and aims: Peroxisome proliferator-activated receptor α (PPARα, NR1C1) is a ligand-activated nuclear receptor involved in the regulation of lipid catabolism and energy homeostasis. PPARα activation induces hepatomegaly and plays an important role in liver regeneration, but the underlying mechanisms remain unclear.
Approach and results: In this study, the effect of PPARα activation on liver enlargement and regeneration was investigated in several strains of genetically modified mice. PPARα activation by the specific agonist WY-14643 significantly induced hepatomegaly and accelerated liver regeneration after 70% partial hepatectomy (PHx) in wild-type mice and Pparafl/fl mice, while these effects were abolished in hepatocyte-specific Ppara-deficient (PparaΔHep ) mice. Moreover, PPARα activation promoted hepatocyte hypertrophy around the central vein area and hepatocyte proliferation around the portal vein area. Mechanistically, PPARα activation regulated expression of yes-associated protein (YAP) and its downstream targets (connective tissue growth factor, cysteine-rich angiogenic inducer 61, and ankyrin repeat domain 1) as well as proliferation-related proteins (cyclins A1, D1, and E1). Binding of YAP with the PPARα E domain was critical for the interaction between YAP and PPARα. PPARα activation further induced nuclear translocation of YAP. Disruption of the YAP-transcriptional enhancer factor domain family member (TEAD) association significantly suppressed PPARα-induced hepatomegaly and hepatocyte enlargement and proliferation. In addition, PPARα failed to induce hepatomegaly in adeno-associated virus-Yap short hairpin RNA-treated mice and liver-specific Yap-deficient mice. Blockade of YAP signaling abolished PPARα-induced hepatocyte hypertrophy around the central vein area and hepatocyte proliferation around the portal vein area.
Conclusions: This study revealed a function of PPARα in regulating liver size and liver regeneration through activation of the YAP-TEAD signaling pathway. These findings have implications for understanding the physiological functions of PPARα and suggest its potential for manipulation of liver size and liver regeneration.
© 2021 American Association for the Study of Liver Diseases.
Conflict of interest statement
CONFLICT OF INTEREST
Nothing to report.
Figures







References
-
- Forbes SJ, Newsome PN. Liver regeneration—mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016;13(8):473–85. - PubMed
-
- Michalopoulos GK. Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65(4):1384–92. - PubMed
-
- Columbano A, Ledda-Columbano GM. Mitogenesis by ligands of nuclear receptors: an attractive model for the study of the molecular mechanisms implicated in liver growth. Cell Death Differ. 2003;10:S19–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

