Molecular basis of mucopolysaccharidosis IVA (Morquio A syndrome): A review and classification of GALNS gene variants and reporting of 68 novel variants
- PMID: 34387910
- PMCID: PMC9291100
- DOI: 10.1002/humu.24270
Molecular basis of mucopolysaccharidosis IVA (Morquio A syndrome): A review and classification of GALNS gene variants and reporting of 68 novel variants
Abstract
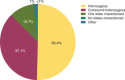
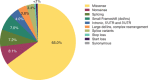
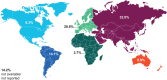
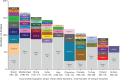
Mucopolysaccharidosis IVA (MPS IVA, Morquio A syndrome) is a rare autosomal recessive lysosomal storage disorder caused by mutations in the N-acetylgalactosamine-6-sulfatase (GALNS) gene. We collected, analyzed, and uniformly summarized all published GALNS gene variants, thus updating the previous mutation review (published in 2014). In addition, new variants were communicated by seven reference laboratories in Europe, the Middle East, Latin America, Asia, and the United States. All data were analyzed to determine common alleles, geographic distribution, level of homozygosity, and genotype-phenotype correlation. Moreover, variants were classified according to their pathogenicity as suggested by ACMG. Including those previously published, we assembled 446 unique variants, among which 68 were novel, from 1190 subjects (including newborn screening positive subjects). Variants' distribution was missense (65.0%), followed by nonsense (8.1%), splicing (7.2%), small frameshift deletions(del)/insertions(ins) (7.0%), intronic (4.0%), and large del/ins and complex rearrangements (3.8%). Half (50.4%) of the subjects were homozygous, 37.1% were compound heterozygous, and 10.7% had only one variant detected. The novel variants underwent in silico analysis to evaluate their pathogenicity. All variants were submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) to make them publicly available. Mutation updates are essential for the correct molecular diagnoses, genetic counseling, prenatal and preimplantation diagnosis, and disease management.
Keywords: GALNS; MPS IVA; Morquio A syndrome; N-acetylgalactosamine-6-sulfate; lysosomal storage disorder; mucopolysaccharidosis IVA.
© 2021 The Authors. Human Mutation published by Wiley Periodicals LLC.
Conflict of interest statement
Moeenaldeen AlSayed has received honorarium and travel reimbursement from BioMarin Pharmaceutical Inc., Shire, and Sanofi Genzyme. Yin‐Hsiu Chien is on the advisory board of Amicus and Sanofi, has received consulting fees from Amicus and Sanofi, has conducted research for Sanofi, and has received honoraria from Biogen, BioMarin Pharmaceutical Inc., Novartis, Sanofi, and Takeda. Roberto Giugliani has been an investigator, consultant, and/or speaker for Abeona, Allevex, Amicus, BioMarin Pharmaceutical Inc., Chiesi, Denali, Idorsia, Inventiva, JCR, Lysogene, Novartis, PassageBio, PTC, RegenxBio, Sanofi‐Genzyme, Sigilon, Sobi, Takeda, and Ultragenyx. Emanuela Izzo and Akashdeep Singh are employees of BioMarin Pharmaceutical Inc. David C. Kasper is an employee of ARCHIMED Life Science GmbH and is a stockholder in ARCHIMED Life Science GmbH. Hsiang‐Yu Lin has been an investigator, consultant, and/or speaker for BioMarin Pharmaceutical Inc., Sanofi‐Genzyme, and Takeda. Shuan‐Pei Lin is on the advisory board of HOS and Shire and has received honoraria from Sanofi Genzyme. Tim Wood is on the advisory board of Amicus‐Fabry. Amelia Morrone has been an investigator, consultant, and/or speaker for BioMarin Pharmaceutical Inc., Sanofi‐Genzyme, and Takeda. The remaining authors declare that there are no conflicts of interest.
Figures

References
-
- Akyol, M. U. , Alden, T. D. , Amartino, H. , Ashworth, J. , Belani, K. , Berger, K. I. , Borgo, A. , Braunlin, E. , Eto, Y. , Gold, J. I. , Jester, A. , Jones, S. A. , Karsli, C. , Mackenzie, W. , Marinho, D. R. , McFadyen, A. , McGill, J. , Mitchell, J. J. , Muenzer, J. , … Consensus Programme Co‐Chairs, M. P. S. (2019). Recommendations for the management of MPS IVA: Systematic evidence‐ and consensus‐based guidance. Orphanet Journal of Rare Diseases, 14, 137. 10.1186/s13023-019-1074-9 - DOI - PMC - PubMed
-
- Bidchol, A. M. , Dalal, A. , Shah, H. , Suryanarayana, S. , Nampoothiri, S. , Kabra, M. , Gupta, N. , Danda, S. , Gowrishankar, K. , Phadke, S. R. , Kapoor, S. , Kamate, M. , Verma, I. C. , Puri, R. D. , Sankar, V. H. , Devi, A. R. , Patil, S. J. , Ranganath, P. , Jain, S. J. , … Girisha, K. M. (2014). GALNS mutations in Indian patients with mucopolysaccharidosis IVA. American Journal of Medical Genetics, Part A, 164A, 2793–2801. 10.1002/ajmg.a.36735 - DOI - PubMed
-
- Bochernitsan, A. N. , Brusius‐Facchin, A. C. , Couto, R. R. , Kubaski, F. , dos Santos Lopes, S. S. , Gondim, C. E. , de Medeiros, P. F. V. , Fischinger, C. , de Souza, M. , Giugliani, R. , & Leistner‐Segal, S. (2018). Spectrum of GALNS mutations and haplotype study in Brazilian patients with mucopolysaccharidosis type IVA. Meta Gene, 16, 77–84. 10.1016/j.mgene.2018.01.008 - DOI
-
- Bunge, S. , Kleijer, W. J. , Tylki‐Szymanska, A. , Steglich, C. , Beck, M. , Tomatsu, S. , Fukuda, S. , Poorthuis, B. J. , Czartoryska, B. , Orii, T. , & Gal, A. (1997). Identification of 31 novel mutations in the N‐acetylgalactosamine‐6‐sulfatase gene reveals excessive allelic heterogeneity among patients with Morquio A syndrome. Human Mutation, 10, 223–232. 10.1002/(SICI)1098-1004(1997)10:3<223::AID-HUMU8>3.0.CO;2-J - DOI - PubMed
-
- Caciotti, A. , Tonin, R. , Mort, M. , Cooper, D. N. , Gasperini, S. , Rigoldi, M. , Parini, R. , Deodato, F. , Taurisano, R. , Sibilio, M. , Parenti, G. , Guerrini, R. , & Morrone, A. (2018). Mis‐splicing of the GALNS gene resulting from deep intronic mutations as a cause of Morquio A disease. BMC Medical Genetics, 19, 183. 10.1186/s12881-018-0694-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

