COVID-19 vaccination during pregnancy: coverage and safety
- PMID: 34389291
- PMCID: PMC8352848
- DOI: 10.1016/j.ajog.2021.08.007
COVID-19 vaccination during pregnancy: coverage and safety
Abstract
Background: Concerns have been raised regarding a potential surge of COVID-19 in pregnancy, secondary to the rising numbers of COVID-19 in the community, easing of societal restrictions, and vaccine hesitancy. Although COVID-19 vaccination is now offered to all pregnant women in the United Kingdom; limited data exist on its uptake and safety.
Objective: This study aimed to investigate the uptake and safety of COVID-19 vaccination among pregnant women.
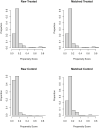
Study design: This was a cohort study of pregnant women who gave birth at St George's University Hospitals National Health Service Foundation Trust, London, United Kingdom, between March 1, 2020, and July 4, 2021. The primary outcome was uptake of COVID-19 vaccination and its determinants. The secondary outcomes were perinatal safety outcomes. Data were collected on COVID-19 vaccination uptake, vaccination type, gestational age at vaccination, and maternal characteristics, including age, parity, ethnicity, index of multiple deprivation score, and comorbidities. Further data were collected on perinatal outcomes, including stillbirth (fetal death at ≥24 weeks' gestation), preterm birth, fetal and congenital abnormalities, and intrapartum complications. Pregnancy and neonatal outcomes of women who received the vaccine were compared with that of a matched cohort of women with balanced propensity scores. Effect magnitudes of vaccination on perinatal outcomes were reported as mean differences or odds ratios with 95% confidence intervals. Factors associated with antenatal vaccination were assessed with logistic regression analysis.
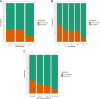
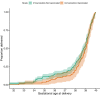
Results: Data were available for 1328 pregnant women of whom 140 received at least 1 dose of the COVID-19 vaccine before giving birth and 1188 women who did not; 85.7% of those vaccinated received their vaccine in the third trimester of pregnancy and 14.3% in the second trimester of pregnancy. Of those vaccinated, 127 (90.7%) received a messenger RNA vaccine and 13 (9.3%) a viral vector vaccine. There was evidence of reduced vaccine uptake in younger women (P=.001), women with high levels of deprivation (ie, fifth quintile of the index of multiple deprivation; P=.008), and women of Afro-Caribbean or Asian ethnicity compared with women of White ethnicity (P<.001). Women with prepregnancy diabetes mellitus had increased vaccine uptake (P=.008). In the multivariable model the fifth deprivation quintile (most deprived) (adjusted odds ratio, 0.10; 95% confidence interval, 0.02-0.10; P=.003) and Afro-Caribbean ethnicity (adjusted odds ratio, 0.27; 95% confidence interval, 0.06-0.85; P=.044) were significantly associated with lower antenatal vaccine uptake, whereas prepregnancy diabetes mellitus was significantly associated with higher antenatal vaccine uptake (adjusted odds ratio, 10.5; 95% confidence interval, 1.74-83.2; P=.014). In a propensity score-matched cohort, the rates of adverse pregnancy outcomes of 133 women who received at least 1 dose of the COVID-19 vaccine in pregnancy were similar to that of unvaccinated pregnant women (P>.05 for all): stillbirth (0.0% vs 0.2%), fetal abnormalities (2.2% vs 2.5%), postpartum hemorrhage (9.8% vs 9.0%), cesarean delivery (30.8% vs 34.1%), small for gestational age (12.0% vs 12.8%), maternal high-dependency unit or intensive care admission (6.0% vs 4.0%), or neonatal intensive care unit admission (5.3% vs 5.0%). Intrapartum pyrexia (3.7% vs 1.0%; P=.046) was significantly increased but the borderline statistical significance was lost after excluding women with antenatal COVID-19 infection (P=.079). Mixed-effects Cox regression showed that vaccination was not significantly associated with birth at <40 weeks' gestation (hazard ratio, 0.93; 95% confidence interval, 0.71-1.23; P=.624).
Conclusion: Of pregnant women eligible for COVID-19 vaccination, less than one-third accepted COVID-19 vaccination during pregnancy, and they experienced similar pregnancy outcomes with unvaccinated pregnant women. There was lower uptake among younger women, non-White ethnicity, and lower socioeconomic background. This study has contributed to the body of evidence that having COVID-19 vaccination in pregnancy does not alter perinatal outcomes. Clear communication to improve awareness among pregnant women and healthcare professionals on vaccine safety is needed, alongside strategies to address vaccine hesitancy. These strategies include postvaccination surveillance to gather further data on pregnancy outcomes, particularly after first-trimester vaccination, and long-term infant follow-up.
Keywords: COVID-19; SARS-CoV-2; coverage; immunization; mRNA; pregnancy; safety vaccine uptake; vaccination; viral vector.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Regarding: COVID-19 vaccination during pregnancy: coverage and safety.Am J Obstet Gynecol. 2022 Mar;226(3):448-449. doi: 10.1016/j.ajog.2021.10.027. Epub 2021 Oct 23. Am J Obstet Gynecol. 2022. PMID: 34699750 Free PMC article. No abstract available.
-
COVID-19 vaccination during pregnancy: coverage and safety, a comment.Am J Obstet Gynecol. 2022 Aug;227(2):370. doi: 10.1016/j.ajog.2022.04.027. Epub 2022 Apr 22. Am J Obstet Gynecol. 2022. PMID: 35469814 Free PMC article. No abstract available.
Similar articles
-
Reductions in stillbirths and preterm birth in COVID-19-vaccinated women: a multicenter cohort study of vaccination uptake and perinatal outcomes.Am J Obstet Gynecol. 2023 May;228(5):585.e1-585.e16. doi: 10.1016/j.ajog.2022.10.040. Epub 2022 Nov 3. Am J Obstet Gynecol. 2023. PMID: 36336084 Free PMC article.
-
Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study.Am J Obstet Gynecol. 2021 Nov;225(5):522.e1-522.e11. doi: 10.1016/j.ajog.2021.05.016. Epub 2021 May 20. Am J Obstet Gynecol. 2021. PMID: 34023315 Free PMC article.
-
Pregnancy and infant outcomes following SARS-CoV-2 infection in pregnancy during delta variant predominance - Surveillance for Emerging Threats to Pregnant People and Infants.Am J Obstet Gynecol MFM. 2024 Feb;6(2):101265. doi: 10.1016/j.ajogmf.2023.101265. Epub 2023 Dec 21. Am J Obstet Gynecol MFM. 2024. PMID: 38135220 Free PMC article.
-
Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy.Nat Commun. 2022 May 10;13(1):2414. doi: 10.1038/s41467-022-30052-w. Nat Commun. 2022. PMID: 35538060 Free PMC article.
-
Differences in pregnancy and perinatal outcomes among symptomatic versus asymptomatic COVID-19-infected pregnant women: a systematic review and meta-analysis.BMC Pregnancy Childbirth. 2021 Dec 1;21(1):801. doi: 10.1186/s12884-021-04250-1. BMC Pregnancy Childbirth. 2021. PMID: 34852783 Free PMC article.
Cited by
-
A socio-ecological exploration to identify factors influencing the COVID-19 vaccine decision-making process among pregnant and lactating women: Findings from Kenya.Vaccine. 2022 Nov 28;40(50):7305-7311. doi: 10.1016/j.vaccine.2022.10.068. Epub 2022 Oct 31. Vaccine. 2022. PMID: 36336529 Free PMC article.
-
An Update on the Status of Vaccine Development for SARS-CoV-2 Including Variants. Practical Considerations for COVID-19 Special Populations.Clin Appl Thromb Hemost. 2022 Jan-Dec;28:10760296211056648. doi: 10.1177/10760296211056648. Clin Appl Thromb Hemost. 2022. PMID: 35167393 Free PMC article. Review.
-
Overview of Neural Tube Defects: Gene-Environment Interactions, Preventative Approaches and Future Perspectives.Biomedicines. 2022 Apr 21;10(5):965. doi: 10.3390/biomedicines10050965. Biomedicines. 2022. PMID: 35625701 Free PMC article. Review.
-
Cross-Sectional Survey of High-Risk Pregnant Women's Opinions on COVID-19 Vaccination.Womens Health Rep (New Rochelle). 2022 Jun 29;3(1):608-616. doi: 10.1089/whr.2022.0006. eCollection 2022. Womens Health Rep (New Rochelle). 2022. PMID: 35814609 Free PMC article.
-
Individual and neighborhood factors associated with being unvaccinated against COVID-19 among pregnant persons.Hum Vaccin Immunother. 2023 Aug 1;19(2):2256042. doi: 10.1080/21645515.2023.2256042. Hum Vaccin Immunother. 2023. PMID: 37697942 Free PMC article.
References
-
- American College of Obstetricians and Gynaecologists Vaccinating pregnant and lactating patients against COVID-19 practice advisory. 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articl... Available at:
-
- Gray K.J., Bordt E.A., Atyeo C., et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. medRxiv. https://doi.org/10.1101/2021.03.07.21253094 Preprint posted online March 8, 2021. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

