In Vivo protection from SARS-CoV-2 infection by ATN-161 in k18-hACE2 transgenic mice
- PMID: 34389403
- PMCID: PMC8352850
- DOI: 10.1016/j.lfs.2021.119881
In Vivo protection from SARS-CoV-2 infection by ATN-161 in k18-hACE2 transgenic mice
Abstract
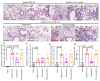
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an infectious disease that has spread worldwide. Current treatments are limited in both availability and efficacy, such that improving our understanding of the factors that facilitate infection is urgently needed to more effectively treat infected individuals and to curb the pandemic. We and others have previously demonstrated the significance of interactions between the SARS-CoV-2 spike protein, integrin α5β1, and human ACE2 to facilitate viral entry into host cells in vitro. We previously found that inhibition of integrin α5β1 by the clinically validated small peptide ATN-161 inhibits these spike protein interactions and cell infection in vitro. In continuation with our previous findings, here we have further evaluated the therapeutic potential of ATN-161 on SARS-CoV-2 infection in k18-hACE2 transgenic (SARS-CoV-2 susceptible) mice in vivo. We discovered that treatment with single or repeated intravenous doses of ATN-161 (1 mg/kg) within 48 h after intranasal inoculation with SARS-CoV-2 lead to a reduction of lung viral load, viral immunofluorescence, and improved lung histology in a majority of mice 72 h post-infection. Furthermore, ATN-161 reduced SARS-CoV-2-induced increased expression of lung integrin α5 and αv (an α5-related integrin that has also been implicated in SARS-CoV-2 interactions) as well as the C-X-C motif chemokine ligand 10 (Cxcl10), further supporting the potential involvement of these integrins, and the anti-inflammatory potential of ATN-161, respectively, in SARS-CoV-2 infection. To the best of our knowledge, this is the first study demonstrating the potential therapeutic efficacy of targeting integrin α5β1 in SARS-CoV-2 infection in vivo and supports the development of ATN-161 as a novel SARS-CoV-2 therapy.
Keywords: ATN-161; Cxcl10; Integrins; SARS-CoV-2; hACE2.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures





References
-
- Control CfD . 2020. Prevention. Interim Clinical Guidance for Management of Patients With Confirmed Coronavirus Disease (COVID-19)
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

