Rates and Patterns of Clonal Oncogenic Mutations in the Normal Human Brain
- PMID: 34389641
- PMCID: PMC8758513
- DOI: 10.1158/2159-8290.CD-21-0245
Rates and Patterns of Clonal Oncogenic Mutations in the Normal Human Brain
Abstract
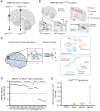
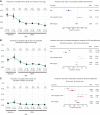
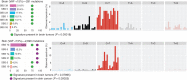
Although oncogenic mutations have been found in nondiseased, proliferative nonneural tissues, their prevalence in the human brain is unknown. Targeted sequencing of genes implicated in brain tumors in 418 samples derived from 110 individuals of varying ages, without tumor diagnoses, detected oncogenic somatic single-nucleotide variants (sSNV) in 5.4% of the brains, including IDH1 R132H. These mutations were largely present in subcortical white matter and enriched in glial cells and, surprisingly, were less common in older individuals. A depletion of high-allele frequency sSNVs representing macroscopic clones with age was replicated by analysis of bulk RNA sequencing data from 1,816 nondiseased brain samples ranging from fetal to old age. We also describe large clonal copy number variants and that sSNVs show mutational signatures resembling those found in gliomas, suggesting that mutational processes of the normal brain drive early glial oncogenesis. This study helps understand the origin and early evolution of brain tumors. SIGNIFICANCE: In the nondiseased brain, clonal oncogenic mutations are enriched in white matter and are less common in older individuals. We revealed early steps in acquiring oncogenic variants, which are essential to understanding brain tumor origins and building new mutational baselines for diagnostics.This article is highlighted in the In This Issue feature, p. 1.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
References
-
- Moore L, Leongamornlert D, Coorens THH, Sanders MA, Ellis P, Dentro SCet al. . The mutational landscape of normal human endometrial epithelium. Nature 2020;580:640–6. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 MH106883/MH/NIMH NIH HHS/United States
- T15 LM007092/LM/NLM NIH HHS/United States
- DP2 AG072437/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 CA215489/CA/NCI NIH HHS/United States
- U54 HD090255/HD/NICHD NIH HHS/United States
- F31 MH124292/MH/NIMH NIH HHS/United States
- K01 AG051791/AG/NIA NIH HHS/United States
- R01 NS032457/NS/NINDS NIH HHS/United States
- R01 CA188228/CA/NCI NIH HHS/United States
- T32 HG002295/HG/NHGRI NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- R01 AG070921/AG/NIA NIH HHS/United States
- P50 CA165962/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

