Astrocyte-derived neurons provide excitatory input to the adult striatal circuitry
- PMID: 34389674
- PMCID: PMC8379996
- DOI: 10.1073/pnas.2104119118
Astrocyte-derived neurons provide excitatory input to the adult striatal circuitry
Abstract
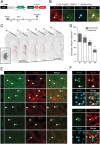
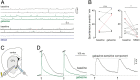
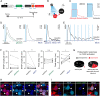
Astrocytes have emerged as a potential source for new neurons in the adult mammalian brain. In mice, adult striatal neurogenesis can be stimulated by local damage, which recruits striatal astrocytes into a neurogenic program by suppression of active Notch signaling (J. P. Magnusson et al., Science 346, 237-241 [2014]). Here, we induced adult striatal neurogenesis in the intact mouse brain by the inhibition of Notch signaling in astrocytes. We show that most striatal astrocyte-derived neurons are confined to the anterior medial striatum, do not express established striatal neuronal markers, and exhibit dendritic spines, which are atypical for striatal interneurons. In contrast to striatal neurons generated during development, which are GABAergic or cholinergic, most adult astrocyte-derived striatal neurons possess distinct electrophysiological properties, constituting the only glutamatergic striatal population. Astrocyte-derived neurons integrate into the adult striatal microcircuitry, both receiving and providing synaptic input. The glutamatergic nature of these neurons has the potential to provide excitatory input to the striatal circuitry and may represent an efficient strategy to compensate for reduced neuronal activity caused by aging or lesion-induced neuronal loss.
Keywords: astrocyte-derived neurogenesis; glutamatergic; neurons; striatum.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Graybiel A. M., Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 31, 359–387 (2008). - PubMed
-
- Alexander G. E., Crutcher M. D., Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci. 13, 266–271 (1990). - PubMed
-
- Kawaguchi Y., Wilson C. J., Augood S. J., Emson P. C., Striatal interneurones: Chemical, physiological and morphological characterization. Trends Neurosci. 18, 527–535 (1995). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

