Structures of tmRNA and SmpB as they transit through the ribosome
- PMID: 34389707
- PMCID: PMC8363625
- DOI: 10.1038/s41467-021-24881-4
Structures of tmRNA and SmpB as they transit through the ribosome
Abstract
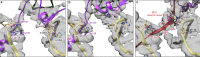
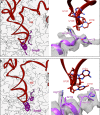
In bacteria, trans-translation is the main rescue system, freeing ribosomes stalled on defective messenger RNAs. This mechanism is driven by small protein B (SmpB) and transfer-messenger RNA (tmRNA), a hybrid RNA known to have both a tRNA-like and an mRNA-like domain. Here we present four cryo-EM structures of the ribosome during trans-translation at resolutions from 3.0 to 3.4 Å. These include the high-resolution structure of the whole pre-accommodated state, as well as structures of the accommodated state, the translocated state, and a translocation intermediate. Together, they shed light on the movements of the tmRNA-SmpB complex in the ribosome, from its delivery by the elongation factor EF-Tu to its passage through the ribosomal A and P sites after the opening of the B1 bridges. Additionally, we describe the interactions between the tmRNA-SmpB complex and the ribosome. These explain why the process does not interfere with canonical translation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


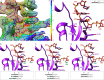
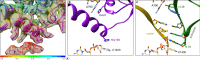


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

