A direct interareal feedback-to-feedforward circuit in primate visual cortex
- PMID: 34389710
- PMCID: PMC8363744
- DOI: 10.1038/s41467-021-24928-6
A direct interareal feedback-to-feedforward circuit in primate visual cortex
Abstract
The mammalian sensory neocortex consists of hierarchically organized areas reciprocally connected via feedforward (FF) and feedback (FB) circuits. Several theories of hierarchical computation ascribe the bulk of the computational work of the cortex to looped FF-FB circuits between pairs of cortical areas. However, whether such corticocortical loops exist remains unclear. In higher mammals, individual FF-projection neurons send afferents almost exclusively to a single higher-level area. However, it is unclear whether FB-projection neurons show similar area-specificity, and whether they influence FF-projection neurons directly or indirectly. Using viral-mediated monosynaptic circuit tracing in macaque primary visual cortex (V1), we show that V1 neurons sending FF projections to area V2 receive monosynaptic FB inputs from V2, but not other V1-projecting areas. We also find monosynaptic FB-to-FB neuron contacts as a second motif of FB connectivity. Our results support the existence of FF-FB loops in primate cortex, and suggest that FB can rapidly and selectively influence the activity of incoming FF signals.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

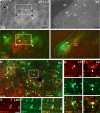
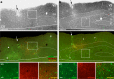

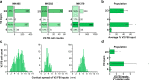

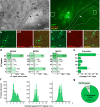

References
-
- Van Essen DC, Maunsell JHR. Hierarchical organization and functional streams in the visual cortex. Trends Neurosci. 1983;6:370–375. doi: 10.1016/0166-2236(83)90167-4. - DOI
-
- Ungerleider, L. G. & Mishkin, M. Two cortical visual systems. in Advances in the Analysis of Visual Behavior (eds Ingle, D. J., Mansfield, J. W., Goodale, M. A.). (MIT Press, 1982).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

